Abstract
Cryptosporidium parvum is an important cause of diarrhea in humans and calves and can persistently infect immunocompromised hosts. Presently, there are no consistently effective parasite-specific drugs for cryptosporidiosis. We hypothesized that neutralizing monoclonal antibodies (MAbs) targeting the apical complex and surface antigens CSL, GP25-200, and P23 could passively immunize against cryptosporidiosis. We recently reported that a formulation of MAbs 3E2 (anti-CSL), 3H2 (anti-GP25-200), and 1E10 (anti-P23) provided significant additive prophylactic efficacy over that of the individual MAbs in neonatal ICR mice. In the present study, these MAbs were evaluated for therapeutic efficacy against persistent infection in adult gamma interferon-depleted SCID mice. 3E2 demonstrated the most significant and consistent therapeutic effect, reducing intestinal infection in two experiments. In one experiment, 3E2 plus 3H2 and 3E2 plus 3H2 plus 1E10 also significantly reduced infection; however, no significant increase in efficacy over 3E2 alone was apparent. The results indicate that anti-CSL MAb 3E2 has highly significant efficacy in reducing, but not eliminating, persistent C. parvum infection.
The apicomplexan parasite Cryptosporidium parvum infects the intestinal tract in humans and calves and other agriculturally important animals and is a leading cause of diarrhea throughout the world (30). In neonates, the elderly, and hosts having congenital or acquired immunodeficiencies, the disease may become chronic and life-threatening (30, 59). Dissemination to extraintestinal sites may occur in immunocompromised hosts and contribute to morbidity (30, 79). While knowledge of the biology of C. parvum has advanced in recent years, there are presently no consistently effective parasite-specific drugs, vaccines, or immunotherapies for cryptosporidiosis (7, 8, 11–13, 18, 19, 21, 22, 24, 32, 36, 37, 39, 40, 47, 51, 53, 57–59, 65–67, 69, 72, 75, 80, 81).
Specific immune responses are known to prevent or terminate C. parvum infection in immunocompetent hosts (reviewed in reference 59). Therefore, passive immunization against C. parvum has been investigated for neonatal and immunocompromised hosts in which inadequate active immune responses predispose to infection and increase its duration and severity (reviewed in references 24 and 59). Early studies with animal models demonstrated that orally administered bovine colostral antibodies produced against whole C. parvum preparations can significantly reduce infection (28, 29, 55, 56, 60, 76). Efficacy of polyclonal antibodies for passive immunotherapy of cryptosporidiosis in humans has also been demonstrated but was inconsistent among studies due largely to patient and treatment variables that complicated experimental designs and interpretation of results (24, 48, 50, 59, 77, 78). Additionally, the efficacy of polyclonal antibody preparations produced against uncharacterized C. parvum antigens may have been limited by their heterogeneity and relatively low content of neutralizing antibodies (16, 59, 83). Nevertheless, these early positive observations provided the rationale to further investigate passive immunization strategies.
We reasoned that passive immunization against C. parvum could be improved through use of high-specific-activity neutralizing monoclonal antibodies (MAbs) to selectively target functionally important antigens of the extracellular infective sporozoite and merozoite stages. To this end, we recently reported the production and characterization of a panel of 126 MAbs (67) against C. parvum apical complex and surface-exposed antigens GP25-200 (3, 64), CSL (64), and P23 (3, 44). Each antigen is expressed by parasites at the infective sporozoite and merozoite stages and has a role in the pathogenesis of infection (3, 39, 52, 64, 67). MAbs determined to have the highest neutralizing activity and to react with distinct epitopes on each antigen were then evaluated, individually and in multiple epitope-specific combinations, for the ability to prevent infection in oocyst-challenged neonatal ICR mice. Anti-CSL MAb 3E2 had the highest protective activity of all MAbs, reducing infection levels by 62 to 92%. 3E2 combined with anti-GP25-200 MAb 3H2 and anti-P23 MAb 1E10 conferred significant additive protection over that provided by the individual MAbs and reduced infection levels by 86 to 93% (67). Complete prevention of infection was observed in up to 40% of mice administered 3E2, alone or in combination with 3H2 and 1E10.
In view of the profound prophylactic efficacy of 3E2 and combinations of MAbs containing 3E2 observed in neonatal ICR mice, the objective of the present study was to determine the therapeutic efficacy of the MAbs against chronic, fulminant gastrointestinal cryptosporidiosis. Because chronic disseminated C. parvum infection does not develop in neonatal ICR or other immunocompetent mice, a fundamentally different adult gamma interferon (IFN-γ)-depleted SCID mouse model was used. 3E2 had the most significant therapeutic effect, consistently reducing intestinal infection in each of two experiments. 3E2 combined with 3H2 and/or IE10 also significantly reduced intestinal and/or biliary infection and fecal oocyst shedding in one experiment. However, the observed reductions were not significantly greater than those in mice treated with 3E2 alone. While the explanation for the apparent lack of increased therapeutic efficacy of the combined MAbs is not entirely clear, the results provide unequivocal evidence that passive immunotherapy with anti-CSL MAb 3E2 can significantly reduce intestinal infection in an immunodeficient-adult-rodent model of persistent cryptosporidiosis.
MATERIALS AND METHODS
Parasites.
The Iowa C. parvum isolate (35) was used in all experiments. Oocysts were obtained from Pleasant Hill Farm (Troy, Idaho) following isolation from experimentally infected newborn Cryptosporidium-free calves as previously described (63). Oocysts were stored in phosphate-buffered saline (PBS) containing 1,000 U of penicillin and 1 mg of streptomycin per ml (4°C) and used within 6 weeks of isolation. Immediately prior to use, oocysts were washed in medium. In vitro excystation (37°C, 0.15% [wt/vol] taurocholate, 2 h) of oocysts used for all mouse inoculations was >90% (53).
Production of MAbs.
Immunoaffinity chromatography purification of P23, GP25-200, and CSL from C. parvum sporozoites and their use for the production of a mouse MAb panel against these antigens have been previously described (67). MAbs 3E2 (anti-CSL), 3H2 (anti-GP25-200), and 1E10 (anti-P23), identified from this panel as having the greatest in vitro and in vivo neutralizing activity of all MAbs generated against each antigen, were produced in quantity by growing hybridomas in bioreactors (Acusyst hollow-fiber cultureware; Cellex, Minneapolis, Minn.) using Iscove’s modified Dulbecco’s medium (HyClone, Logan, Utah). Bioreactor-derived MAbs were dialyzed (4°C) against PBS and concentrated by tangential-flow filtration (PLMK TFF cartridge; exclusion limits of 300 kDa for immunoglobulin M [IgM] MAbs 3E2 and 3H2 and 30 kDa for IgG1 MAb 1E10; Millipore, Bedford, Mass.). After processing, the MAbs were evaluated for retention of sporozoite and merozoite reactivity by immunofluorescence assay (10, 64) and for antigen recognition by Western immunoblotting using previously described methods (60, 64). Mouse IgM and IgG1 isotype control MAbs of irrelevant specificity, previously shown to be unreactive with C. parvum (9, 52, 64, 67), were also produced in bioreactors (CellMax artificial capillary cell culture system; Cellco, Germantown, Md.) using serum-free medium (Gibco Life Technologies), concentrated by ultrafiltration (Centriprep; 30-kDa exclusion limit; Millipore), and dialyzed (exclusion limit of 12 to 14 kDa) against PBS (4°C). All MAb concentrations were determined by radial immunodiffusion (Binding Site, San Diego, Calif.).
Evaluation of MAbs for therapeutic efficacy.
Two experiments were performed to determine the effect of 3E2, 3H2, and 1E10, individually and in combinations, against chronic C. parvum infection in adult female C.B.17/Icr Tac-SCID mice (Taconic, Germantown, N.Y.). For both experiments, mice were housed in sterile, filter-top microisolator cages with autoclaved food and water and allowed to acclimate for 2 days following arrival at 4 weeks of age. Mice were then injected intraperitoneally, once daily for 2 days, with 105 neutralizing units of purified rat anti-mouse IFN-γ MAb from rat-mouse heterohybridoma cell line R46A2 (20). At 5 weeks of age, each mouse was inoculated with 107 oocysts by gastric intubation using a 22-gauge feeding tube. Four weeks after inoculation, fecal pellets were collected from individual mice to confirm infection and to determine the number of oocysts per milligram of feces using previously described methods (54). Mice were defined as being persistently infected based on the presence of oocysts in feces 4 weeks following inoculation. Individual persistently infected mice were weighed on the day MAb treatment was initiated and twice weekly thereafter for the duration of each experiment.
In experiment 1, 98 infected mice were randomly assigned to one of seven groups, each containing seven mice shedding oocysts at a level above the median number per milligram of feces and seven mice shedding oocysts at a level below the median. Groups consisted of mice given 3E2, 3H2, 1E10, 3E2 plus 3H2, 3E2 plus 1E10, 3E2 plus 3H2 plus 1E10, or IgG1 plus IgM isotype control MAbs. At day 39 post-oocyst inoculation, all mice were given cimetidine (60 mg/kg of body weight per os) and were re-treated 12 and 24 h later in an attempt to reduce the degradative effects of gastric acidity and proteases on orally administered antibodies (76, 83). This regimen was empirically determined after a dose-response evaluation of cimetidine in adult ICR mice, and observations of the maximum elevation of gastric acidity achievable 12 h following treatment yielded pH 4 to 5.5. Twelve hours after cimetidine treatment, groups of 14 mice each were administered their designated MAb preparations by gastric intubation. MAb preparations were formulated in PBS containing 1% (wt/vol) ovalbumin and cimetidine (4 mg/ml), with each MAb present at a final concentration of 5 mg/ml, and administered in a volume of 250 μl per mouse. Every 12 h thereafter for 21 days, mice were treated identically with the MAb preparations for a total of 42 treatments. One mouse each in the 3H2 and 3E2-plus-3H2 groups was euthanized in extremis following aspiration of MAbs shortly after treatment commenced and were therefore excluded from the study. Throughout the treatment period, mice were housed two per cage on raised wire mesh flooring to allow passage of feces into a moist collection tray. Feces excreted over a 24-h period were collected on the day prior to initiating treatment and on treatment days 3, 5, 7, 10, 12, 14, 17, 19, and 21 and placed in PBS containing 0.2% (wt/vol) azide. The total number of oocysts recovered in 24-h collections at each time point from mice in each group was then evaluated by immunofluorescence microscopy using C. parvum oocyst-specific MAb 4D3 (60). Bedding was removed from cages prior to each 24-h fecal collection, after which new bedding was provided. All mice were euthanized by CO2 asphyxiation 10 h after the final treatment. Sections of the gall bladder-common bile duct junction, pyloric region of the stomach, terminal ileum, cecum, and proximal colon were collected from the same anatomic site in each mouse and processed for histopathology. Slides were coded by one investigator and examined histologically by a second investigator, without knowledge of treatment group, for C. parvum stages in the mucosal epithelium. Scores of 0, 1, 2, or 3 (0, no infection; 1, <33% of mucosa infected; 2, 33 to 66% of mucosa infected; 3, >66% of mucosa infected) were assigned to longitudinal sections from each tissue using standardized methods as previously described (60, 63). Scores obtained from equivalent lengths of the terminal ileum, cecum, and proximal colon for each mouse were summed to obtain an intestinal infection score based on the consistency between significance conclusions from statistical analysis of either summed or individual scores for these intestinal regions (60, 67).
In experiment 2, 100 infected mice were randomly assigned to one of five groups, each containing 20 mice. Groups were given 3E2, 3E2 plus 3H2, 3E2 plus 1E10, 3E2 plus 3H2 plus 1E10, or IgM (10 mice) and IgG1 plus IgM (10 mice) isotype control MAbs. At day 35 post-oocyst inoculation, all mice were pretreated with cimetidine as described for experiment 1. Twelve hours later, and every 12 h thereafter for 21 days, mice were administered their designated MAb preparations for a total of 42 treatments. MAb preparations were formulated as described for experiment 1 and administered in a volume of 250 μl per mouse except that each MAb was present at a final concentration of 4 mg/ml. Mice were housed on standard autoclaved bedding. Fecal pellets from individual mice were collected on the day prior to initiating treatment and on treatment days 4, 11, and 18, for a total of three posttreatment samples. Pellets were placed in PBS containing 0.2% (wt/vol) azide immediately after collection and used to determine the number of oocysts per milligram of feces as previously described (60). All mice were euthanized 10 h after the final treatment, and tissue sections were collected, coded, and scored histologically without knowledge of treatment group as described for experiment 1.
Mean tissue infection scores, fecal oocyst production, and body weights were evaluated by analysis of variance using the General Linear Models Program of SAS (SAS/STAT, release 6.03, user’s guide; SAS Institute, Cary, N.C., 1988). Probability values less than 0.05 were considered significant. Because no significant differences in values for these parameters between the IgM and IgG1-plus-IgM isotype control-treated mice in experiment 2 were identified, the groups were combined for subsequent statistical comparisons.
RESULTS
Characterization of MAbs following bioreactor production and processing.
MAbs were produced in bioreactors to obtain the quantities required for therapeutic studies in pure form. Production and processing conditions did not affect reactivity with sporozoites and merozoites in immunofluorescence assays, in vitro sporozoite-neutralizing activity, or in vivo neutralizing activity in oocyst-challenged neonatal ICR mice. Western blot reactivities of the MAbs were also unaffected, being indistinguishable from those reported previously (67).
Passive immunotherapy with anti-CSL MAb 3E2 alone has highly significant efficacy, with consistent therapeutic predictability, against persistent intestinal infection with C. parvum.
(i) Reduction of tissue infection levels.
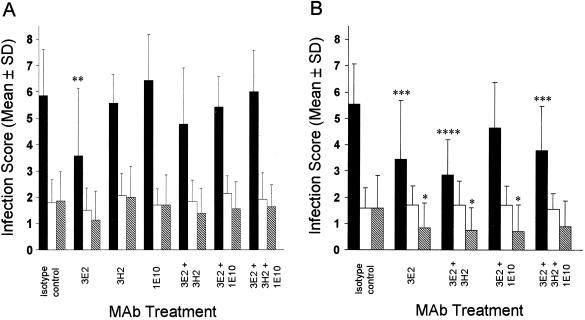
In experiment 1, treatment with 3E2 resulted in a highly significant ∼39% reduction of intestinal infection levels compared to isotype control treatment (Fig. 1A). Pyloric and biliary infection levels were also lower in 3E2-treated mice, but the reductions were not statistically significant. In experiment 2, 3E2 efficacy against intestinal infection was confirmed: 3E2 reduced infection by ∼38% compared to the isotype control (Fig. 1B). In addition, biliary infection levels were ∼46% lower in 3E2-treated mice (Fig. 1B). Unlike treatment with 3E2, treatment with either 3H2 or 1E10 had no significant effect on infection in any anatomic site examined (Fig. 1A).
FIG. 1.
Therapeutic efficacy of MAbs 3E2, 3H2, and 1E10, individually and in combinations, against persistent C. parvum infection in the intestinal tract (solid bars), pylorus (open bars), and gall bladder-common bile duct (hatched bars) in experiments 1 (A) and 2 (B). *, **, ***, and ****, P < 0.05, 0.01, 0.0025, and 0.0001, respectively, compared to results for the isotype control MAb-treated group. Error bars, standard deviations (SD).
(ii) Reduction of fecal oocyst production.
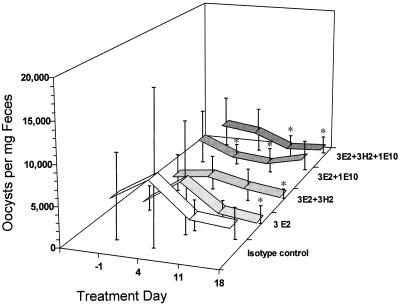
To provide a quantitative index of parasite load, experiment 1 was designed to determine the total number of oocysts excreted by mice in each group. However, during the experiment it was observed that mice housed on raised wire mesh flooring without bedding frequently fed, defecated, and huddled in one area of the cage. This behavior resulted in the presence of variable amounts of food and debris in fecal trays and desiccation of fecal pellets, influencing oocyst recovery and precluding accurate quantitation. Statistical comparisons of the mean total number of oocysts recovered and detected in 24-h collections prior to and after all MAb treatments were nevertheless performed and revealed (i) significantly higher oocyst numbers in the isotype control group on days 5, 17, 19, and 21 than on day 3 (P < 0.05), (ii) a significantly higher number of oocysts in the 3H2 group on day 21 than on any preceding time point (P < 0.05), (iii) a significantly lower number of oocysts in the 3E2-plus-3H2-plus-1E10 group on day 5 than on day 14 (P < 0.05), and (iv) no significant differences in the 3E2, 1E10, 3E2-plus-3H2, and 3E2-plus-1E10 groups at any time point. In experiment 2, mice were housed in standard cages and bedding and fecal pellets were collected directly from individual mice to facilitate accurate oocyst quantitation. Statistical comparison of the mean number of oocysts per milligram of feces prior to and after treatment with 3E2 indicated a significant ∼55% reduction in oocyst production within the group by day 18 of treatment (Fig. 2). Comparison of fecal oocyst production by mice prior to and after treatment with isotype control MAbs revealed no significant differences within the group at any time point examined (Fig. 2).
FIG. 2.
Oocyst-shedding patterns in persistently infected SCID mice treated with anti-C. parvum or isotype control MAbs. The mean number of fecal oocysts was significantly (*) lower in mice treated with 3E2 (day 18, P < 0.05), 3E2 plus 3H2 (day 18, P < 0.025), 3E2 plus 1E10 (day 4, P < 0.01, and day 11, P < 0.005), or 3E2 plus 3H2 plus 1E10 (days 11 and 18, P < 0.005) than the pretreatment (day −1) values for each group. Error bars, standard deviations.
(iii) Effect on body weight.
In experiment 1, comparison of the mean body weights of mice prior to and after treatment with individual MAbs 3E2, 3H2, and 1E10 or isotype control MAbs revealed no significant changes within each group during the treatment period. However, in experiment 2, comparison of the mean body weights of isotype control mice prior to and after treatment revealed significant weight loss beginning at treatment day 11 (P < 0.0005) and continuing until day 17 (P < 0.025). In contrast, no significant changes in the mean body weight of 3E2-treated mice were observed during the treatment period. From day 14 to the end of the experiment, the mean body weight of 3E2-treated mice was greater than that of the isotype control mice, and these differences approached significance.
Passive immunotherapeutic efficacy of MAb 3E2, 3H2, and 1E10 combinations against persistent C. parvum infection.
The rationale for selection of the MAb combinations evaluated was twofold. First, 3E2 was considered an essential component based on results of previous studies demonstrating its superior activity against C. parvum (64, 67). Second, of all possible combinations, those selected had consistently demonstrated the greatest prophylactic efficacy against oocyst challenge (67).
(i) Effect on tissue infection levels.
In experiment 1, each of the three combinations evaluated had no significant effect on infection levels, although intestinal and biliary scores in mice treated with 3E2 plus 3H2 were lower than those in the isotype control group mice (Fig. 1A). Therefore, the second experiment was performed to determine if the apparent reduction of infection by 3E2 plus 3H2 was significant and to further investigate the observation that no increase in efficacy over 3E2 alone was conferred by any of the combinations. Larger treatment groups were used to increase the power of statistical analyses. Mice were also housed conventionally on standard bedding for the duration of experiment 2. In this experiment, treatment with 3E2 plus 3H2 plus 1E10 or 3E2 plus 3H2 significantly reduced intestinal infection levels by ∼31 and ∼48%, respectively, compared to the isotype control group treatment (Fig. 1B). Mice treated with 3E2 plus 3H2 or 3E2 plus 1E10 also had significantly lower biliary infection levels, representing ∼53 to ∼56% reductions (Fig. 1B). Mice treated with 3E2 plus 3H2 had lower intestinal and biliary infection scores than mice treated with 3E2 alone; however, the reductions observed with this and the other combinations evaluated were not statistically different from those observed with 3E2 alone (Fig. 1B).
(ii) Effect on fecal oocyst production.
Based on comparison of the mean numbers of oocysts per milligram of feces prior to and after treatment in experiment 2, all MAb combinations significantly reduced oocyst production within each group over time (Fig. 2). Significant reductions were observed as early as day 4 (∼46%) and continued to day 11 (∼57%) in mice treated with 3E2 plus 1E10 but were no longer evident at day 18 (Fig. 2). In mice treated with 3E2 plus 3H2, a significant ∼59% reduction was evident by day 18 (Fig. 2). Oocyst production in mice treated with 3E2 plus 3H2 plus 1E10 was significantly reduced by day 11 (∼69%), and this reduction continued until day 18 (∼59%) (Fig. 2).
(iii) Effect on body weight.
In experiment 1, comparison of the mean body weights of mice prior to and after treatment with each MAb combination revealed no significant changes within each group during the treatment period. On day 14 of experiment 1, mice treated with 3E2 plus 1E10 or 3E2 plus 3H2 were observed to have significant weight reductions compared to the isotype control group (P < 0.005). In experiment 2, the mean body weights of mice treated with each MAb combination did not change significantly during the treatment period, unlike those of the isotype control group, which lost weight. Beginning on day 11 and continuing to the end of the experiment, the mean body weights of mice treated with 3E2 plus 3H2 or 3E2 plus 3H2 plus 1E10 were significantly greater than those of the isotype control mice (P < 0.05). On days 11 and 14, the mean body weights of mice treated with 3E2 plus 1E10 were also significantly greater than those of the isotype control mice (P < 0.025).
DISCUSSION
Passive immunization by locally delivered antibody is a valid means for preventing or treating infection by a variety of enteric and other pathogens (6, 16, 17, 31, 38, 70, 71, 82, 83). Its feasibility has been extended for use in cryptosporidiosis by results of initial studies evaluating antibodies generated against uncharacterized whole C. parvum preparations (9, 54, 60). In the present study, we hypothesized that passive immunotherapy against persistent C. parvum infection could be enhanced by targeting antigens known to function in the infection process. Because surface-exposed and apical complex antigens are critical to infection, they are opportune targets for immunization against C. parvum (7, 10, 18, 34, 36, 37, 39, 42, 46, 47, 53, 57, 61, 62, 64, 66, 67, 74, 80) as well as other apicomplexan parasites (5, 14, 15, 23, 25, 46, 68). Therefore, neutralizing MAbs against the surface pellicle antigen P23, the apical glycoprotein antigen complex GP25-200, and the apical complex glycoprotein CSL were prepared for therapeutic evaluation.
Several adult rodent models have been developed for characterization of immune responses to C. parvum and evaluation of potential therapeutic agents (1, 2, 20, 33, 72, 73; earlier studies reviewed in references 41 and 59). C57BL/6 mice having functional B and T lymphocytes but disruption of the IFN-γ gene develop acute overwhelming C. parvum infection (73). Severe small-intestine infection and extensive mucosal destruction result in rapidly progressive wasting and death within 2 to 3 weeks; therefore, dissemination to the biliary tract does not occur and only mild pyloric infection is observed (73). IFN-γ gene knockout mice are being used to further define C. parvum-specific T- and B-lymphocyte responses and to distinguish protective from nonprotective active immune responses (73). These mice also provide a sensitive model to test potential therapeutic agents for acute life-threatening C. parvum infection (33, 73). Athymic nude mice used in early studies demonstrated the central role of T lymphocytes and IFN-γ in controlling C. parvum infection (41, 59). More recently, athymic mouse strains have been used to phenotype endogenous intestinal intraepithelial lymphocyte populations involved in resistance to C. parvum (1). SCID mice lack functional T and B lymphocytes and have been useful for defining the role of specific cytokines and adoptively transferred peripheral and intestinal intraepithelial lymphocyte populations in controlling C. parvum infection (2, 41, 59). SCID mice have also been used for evaluation of passive immunization strategies (24, 41, 59). Of these models, adult SCID mice, predisposed to disseminated C. parvum infection by anti-IFN-γ MAb treatment (20, 72), were selected for therapeutic evaluation of MAbs recently shown to protect against primary infection (67). Chronic C. parvum infection can be consistently established in such mice, providing a stringent model that is especially suitable for evaluation of passive immunotherapy because of its independence from the influence of specific cellular or humoral host immune responses (20, 54, 60, 72, 76). Unlike the neonatal ICR mouse model widely used for initial studies to evaluate potential anticryptosporidial agents, adult SCID mice have a physiologically and anatomically mature gastrointestinal system through which MAbs must survive transit. In addition, neutrophilic enterocolitis with crypt microabscessation and hyperplasia occur in chronically infected SCID mice but not in neonatal ICR mice (60, 76). The physical presence of exudate, as well as inflammatory proteases, may impede delivery of MAbs to mucosal surfaces where C. parvum propagates. Finally, in SCID mice C. parvum may disseminate to the hepatobiliary tract and pylorus after the infection becomes chronic. Such extraintestinal sites may then provide reservoirs of parasites that can reinfect intestinal mucosa (54, 60, 76).
Treatment with anti-CSL MAb 3E2 alone resulted in the most significant and consistent therapeutic effect, reducing both intestinal infection and fecal oocyst production. These observations parallel its previously reported sporozoite-neutralizing activity in vitro and ability to passively protect neonatal mice against oocyst challenge (39, 67), which are greater than those of all other MAbs against C. parvum that we have produced (27, 52, 55, 61, 62, 64, 67). In one experiment, 3E2 in combination with either anti-GP25-200 MAb 3H2 or 3H2 and anti-P23 MAb 1E10 also significantly reduced intestinal infection and oocyst production, while 3E2 in combination with 1E10 was only effective in reducing oocyst production. The lower intestinal infection score for mice treated with the combination of 3E2 plus 3H2 versus that for mice treated with 3E2 alone was similar to previous results for neonatal ICR mice administered this combination prophylactically (67). However, unlike the results for neonatal mice, the difference was not statistically significant. We also observed in this experiment that biliary infection in mice treated with 3E2, 3E2 plus 3H2, or 3E2 plus 1E10 was significantly reduced. However, because the biliary tract is anatomically sheltered from orally delivered MAbs and because ascending infection to this site is a sequela to chronic gastrointestinal infection (30, 41, 79), the observed reductions are considered secondary treatment effects associated with lower gastrointestinal infection levels.
Because individual and combined MAb concentrations tested in the present study were similar to those evaluated in prophylaxis studies (67), the results suggest no additive therapeutic effect of the MAb combinations over 3E2 alone. These observations likely reflect the fundamental model differences in evaluating treatment efficacy of MAbs administered after infection becomes chronic and disseminated in immunodeficient adult mice versus the prophylactic efficacy of MAbs administered concurrently with oocyst challenge in normal neonatal mice. The enhanced efficacy of MAb combinations may be an advantageous property which is more applicable for prevention of infection than treatment of chronic infection. Another possible explanation is that CSL may have a pivotal role in both initiation of primary infection and perpetuation of chronic infection while GP25-200 and/or P23 may function principally in initiating infection. If so, differential expression of these antigens by sexual and asexual parasite stages, including autoinfective sporozoites and merozoites, would be expected during chronic infection and could explain differences in treatment versus prophylactic effects.
The apparent lack of efficacy of MAb combinations observed in experiment 1 could be accounted for by the following variables. In this experiment, treatment began on day 39 postinfection, whereas in experiment 2 treatment was initiated on day 35 postinfection; therefore, infection in experiment 1 was likely more advanced. Higher infection scores in both the control and treatment groups observed in experiment 1 confirmed that infection had indeed progressed beyond that present in experiment 2, a finding consistent with the asynchronous replication of C. parvum and completion of each cycle in 48 to 72 h (30, 75). Higher intestinal infection levels in experiment 1 may also account for the observation that secondary biliary infection was not reduced in 3E2-treated mice, unlike what was found in experiment 2. It is also possible that infection levels in experiment 1 exceeded the threshold for efficacy detection of MAb combinations in the treatment and evaluation regimens used. Related to this, it is highly probable that the smaller group sizes in experiment 1 (13 to 14 mice/group) yielded mean infection scores that were not representative of a larger treatment population such as that used in experiment 2 (20 mice/group). Despite the higher starting dose in experiment 1, pharmacologically active levels of the combined MAbs reaching the intestinal mucosa in some mice may have been lower than in experiment 2 due to increased proteolytic degradation. This possibility is suggested by lesion progression and the likely increase in activity of inflammatory cell and plasma-derived proteases in mucosal exudates due to the longer duration of infection prior to treatment in experiment 1. A final possible explanation, that there was antagonism between the combined MAbs at the higher concentrations used in experiment 1, is inconsistent with the results of previous studies (67). However, antagonism cannot be excluded without additional pharmacokinetics studies (26). Despite the preceding variables, 3E2 significantly reduced intestinal infection in both experiments, further substantiating its activity against C. parvum. Importantly, none of the MAbs evaluated had any significant effect on gastric infection. This is most likely due to the acidic gastric environment, effectively reducing the affinity of, or eliminating, antibody-antigen binding. Nevertheless, reduction of intestinal infection confirmed that sufficient quantities of MAbs can survive transit through the adult gastrointestinal environment to mediate neutralization.
Based on studies of related apicomplexan parasites, therapeutic antibodies for cryptosporidiosis would be expected to bind extracellular stages and prevent their attachment and invasion (43, 49, 59) or disrupt intracellular development of bound stages which retain the ability to invade (43, 45, 49, 59). However, knowledge of specific mechanisms by which antibodies mediate neutralization of C. parvum is currently limited and is needed to further develop immunization strategies (24, 59, 80). Neutralization by 3E2 involves binding of CSL and occurrence of the circumsporozoite precipitate (CSP)-like reaction, after which sporozoites and merozoites are rendered noninfective (64). Because 3E2 is of the IgM isotype and recognizes a repetitive epitope in CSL, its unique ability to elicit the neutralizing CSP-like reaction reflects multivalent binding. We have recently reported that CSL contains a sporozoite ligand which is involved in specific binding to a receptor on the surface of human and bovine intestinal epithelial cells (39, 40). These findings can explain the biological basis for the treatment efficacy of 3E2 observed in the present study and the prophylactic efficacy observed in previous studies (67). Mechanisms of neutralization by 3H2 and 1E10 are the subject of ongoing studies. While 3H2 does not elicit the CSP-like reaction, it recognizes apical complex and surface pellicle antigens (67) and likely targets attachment and/or invasion. Neutralization by 1E10 may involve impairment of sporozoite and merozoite locomotion mediated by P23 deposition and surface translocation during gliding motility and invasion (4, 27). The important role of P23 in the infection process has been demonstrated by the observation that orally administered monospecific immune bovine colostrum prepared against recombinant P23 prevented diarrhea in oocyst-challenged calves and reduced oocyst shedding by ∼600-fold (53).
In summary, we conclude that passive immunotherapy using MAb 3E2 to target CSL has highly significant efficacy in reducing the severity of intestinal infection in an immunodeficient adult rodent model of persistent cryptosporidiosis. While the results suggest that the polyvalent neutralizing MAb combinations evaluated may not significantly increase efficacy over that of individual MAbs after infection becomes chronic, one rationale for the use of MAb combinations stems from the prolonged treatment regimens considered necessary to ameliorate infection in immunodeficient hosts (9, 24, 48, 54, 59, 60, 76–78). If antigenic variation in C. parvum occurs, therapy directed at more than one epitope may reduce the selection and emergence of variant C. parvum subpopulations, which would be expected over time and which would complicate treatment.
It is important to note that, despite the efficacy of 3E2, alone or in combinations with 3H2 and 1E10, in no case was infection completely eradicated in every intestinal and extraintestinal region examined in individual mice. Other investigators have reported that high doses of the aminoglycoside paromomycin administered to anti-IFN-γ-treated SCID mice (72) or IFN-γ gene knockout mice (33) also failed to completely eradicate C. parvum infection in the pylorus, ileum, cecum, or colon of any mice. These findings and the observed inefficacy on pyloric infection highlight the limitations that must be overcome before MAb therapy can be effectively used against persistent cryptosporidiosis. In such cases, gastric pH must be stably neutralized and pyloric infection must be cleared or it is probable that reinfection of the intestinal tract will occur from this site or the biliary tract.
In light of these factors, MAb therapy in immunocompromised humans may presently have applicability when infection is diagnosed promptly in the acute stage and aggressive treatment of extended duration is immediately instituted. If partial restoration of CD4+ T-lymphocyte counts can also be achieved, by highly active antiretrovirus therapy in AIDS patients or by interruption of treatment in chemically immunosuppressed patients, MAb therapy would have a higher probability of success. Finally, results of the present and previous studies (67), taken together, suggest that MAb therapy could have immediate application in certain cases of cryptosporidiosis in immunocompetent, elderly, and neonatal patients in which parasite-specific treatment may be medically indicated.
Acknowledgments
This work was supported by Public Health Service grant AI 30223 from the National Institutes of Health, Bethesda, Md., and U.S. Department of Agriculture NRICGP grants 94-37204-0496, 99-35204-8533, and 2000-35204-09960. Costs for bioreactor production of MAbs 3E2, 3H2, and 1E10 by the National Cell Culture Center were subsidized through a Cooperative Agreement Award from the National Center for Research Resources, National Institutes of Health. Deborah A. Schaefer was supported in part by funds from the Microbiology and Immunology Graduate Program, University of Arizona.
We thank Deborah Dalton, Beth Auerbach-Dixon, Kathryn Huey Tubman, and Jessica Chodos for excellent technical assistance and E. Havell for providing R46A2 hybridoma cells as a source of anti-mouse IFN-γ antibody.
REFERENCES
- 1.Adjei, A. A., J. T. Jones, and F. J. Enriquez. 2000. Differential intraepithelial lymphocyte phenotypes following Cryptosporidium parvum challenge in susceptible and resistant athymic strains of mice. Parasitol. Int. 49:119–129. [DOI] [PubMed] [Google Scholar]
- 2.Adjei, A. A., A. K. Shrestha, M. Castro, and F. J. Enriquez. 2000. Adoptive transfer of immunity with intraepithelial lymphocytes in Cryptosporidium parvum-infected severe combined immunodeficient mice. Am. J. Med. Sci. 320:304–309. [DOI] [PubMed] [Google Scholar]
- 3.Arrowood, M. J., J. R. Mead, J. L. Mahrt, and C. R. Sterling. 1989. Effects of immune colostrum and orally administered antisporozoite monoclonal antibodies on the outcome of Cryptosporidium parvum infections in neonatal mice. Infect. Immun. 57:2283–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arrowood, M. J., C. R. Sterling, and M. C. Healey. 1991. Immunofluorescent microscopic visualization of trails left by gliding Cryptosporidium parvum sporozoites. J. Parasitol. 77:315–317. [PubMed] [Google Scholar]
- 5.Augustine, P. C. 2000. Cellular invasion by avian Eimeria species. Avian Poultry Biol. Rev. 11:113–122. [Google Scholar]
- 6.Barekzi, N. A., K. A. Poelstra, A. G. Felts, I. A. Rojas, J. B. Slunt, and D. W. Grainger. 1999. Efficacy of locally delivered polyclonal immunoglobulin against Pseudomonas aeruginosa peritonitis in a murine model. Antimicrob. Agents Chemother. 43:1609–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnes, D. A., A. Bonnin, J. X. Huang, L. Gousset, J. Wu, J. Gut, P. Doyle, J. F. Dubremetz, H. Ward, and C. Petersen. 1998. A novel multi-domain mucin-like glycoprotein of Cryptosporidium parvum mediates invasion. Mol. Biochem. Parasitol. 96:93–110. [DOI] [PubMed] [Google Scholar]
- 8.Benbow, J. W., E. L. Bernberg, A. Korda, and J. R. Mead. 1998. Synthesis and evaluation of dinitroanilines for treatment of cryptosporidiosis. Antimicrob. Agents Chemother. 42:339–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bjorneby, J. M., B. D. Hunsaker, M. W. Riggs, and L. E. Perryman. 1991. Monoclonal antibody immunotherapy in nude mice persistently infected with Cryptosporidium parvum. Infect. Immun. 59:1172–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bjorneby, J. M., M. W. Riggs, and L. E. Perryman. 1990. Cryptosporidium parvum merozoites share neutralization-sensitive epitopes with sporozoites. J. Immunol. 145:298–304. [PubMed] [Google Scholar]
- 11.Blagburn, B. L., K. L. Drain, T. M. Land, R. G. Kinard, P. H. Moore, D. S. Lindsay, D. A. Patrick, D. W. Boykin, and R. R. Tidwell. 1998. Comparative efficacy evaluation of dicationic carbazole compounds, nitazoxanide, and paromomycin against Cryptosporidium parvum infections in a neonatal mouse model. Antimicrob. Agents Chemother. 42:2877–2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blagburn, B. L., and R. Soave. 1997. Prophylaxis and chemotherapy: human and animal, p.111–128. In R. Fayer (ed.), Cryptosporidium and cryptosporidiosis. CRC Press, Boca Raton, Fla.
- 13.Bonafonte, M. T., L. M. Smith, and J. R. Mead. 2000. A 23-kDa recombinant antigen of Cryptosporidium parvum induces a cellular immune response on in vitro stimulated spleen and mesenteric lymph node cells from infected mice. Exp. Parasitol. 96:32–41. [DOI] [PubMed] [Google Scholar]
- 14.Brown, W. C., and G. H. Palmer. 1999. Designing blood-stage vaccines against Babesia bovis and Babesia bigemina. Parasitol. Today 15:275–281. [DOI] [PubMed] [Google Scholar]
- 15.Carruthers, V. B., and L. D. Sibley. 1997. Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur. J. Cell Biol. 73:114–123. [PubMed] [Google Scholar]
- 16.Casadevall, A. 1999. Passive antibody therapies: progress and continuing challenges. Clin. Immunol. 93:5–15. [DOI] [PubMed] [Google Scholar]
- 17.Casadevall, A., W. Cleare, M. Feldmesser, A. Glatman-Freedman, D. L. Goldman, T. R. Kozel, N. Lendvai, J. Mukherjee, L. Pirofski, J. Rivera, A. L. Rosas, M. D. Scharff, P. Valadon, K. Westin, and Z. Zhong. 1998. Characterization of a murine monoclonal antibody to Cryptococcus neoformans polysaccharide that is a candidate for human therapeutic studies. Antimicrob. Agents Chemother. 42:1437–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cevallos, A. M., N. Bhat, R. Verdon, D. H. Hamer, B. Stein, S. Tzipori, M. E. A. Pereira, G. T. Keusch, and H. D. Ward. 2000. Mediation of Cryptosporidium parvum infection in vitro by mucin-like glycoproteins defined by a neutralizing monoclonal antibody. Infect. Immun. 68:5167–5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cevallos, A. M., X. Zhang, M. K. Waldor, S. Jaison, X. Zhou, S. Tzipori, M. R. Neutra, and H. D. Ward. 2000. Molecular cloning and expression of a gene encoding Cryptosporidium parvum glycoproteins gp40 and gp15. Infect. Immun. 68:4108–4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen, W., J. A. Harp, A. G. Harmsen, and E. A. Havell. 1993. Gamma interferon functions in resistance to Cryptosporidium parvum infection in severe combined immunodeficient mice. Infect. Immun. 61:3548–3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen, X. M., and N. F. LaRusso. 2000. Mechanisms of attachment and internalization of Cryptosporidium parvum to biliary and intestinal epithelial cells. Gastroenterology 118:368–379. [DOI] [PubMed] [Google Scholar]
- 22.Coombs, G. H. 1999. Biochemical peculiarities and drug targets in Cryptosporidium parvum: lessons from other coccidian parasites. Parasitol. Today 15:333–338. [DOI] [PubMed] [Google Scholar]
- 23.Coppel, R. L., G. V. Brown, and V. Nussenzweig. 1998. Adhesive proteins of the malaria parasite. Curr. Opin. Microbiol. 1:472–481. [DOI] [PubMed] [Google Scholar]
- 24.Crabb, J. H. 1998. Antibody-based immunotherapy of cryptosporidiosis. Adv. Parasitol. 40:121–149. [DOI] [PubMed] [Google Scholar]
- 25.Dubremetz, J. F., N. Garcia-Réguet, V. Conseil, and M. N. Fourmaux. 1998. Apical organelles and host-cell invasion by Apicomplexa. Int. J. Parasitol. 28:1007–1013. [DOI] [PubMed] [Google Scholar]
- 26.Eliopoulos, G. M., and R. C. Moellering. 1996. Antimicrobial combinations, p.330–396. In V. Lorian (ed.), Antibiotics in laboratory medicine, 4th ed., The Williams and Wilkins Co., Baltimore, Md.
- 27.Enriquez, F. J., and M. W. Riggs. 1998. Role of immunoglobulin A monoclonal antibodies against P23 in controlling murine Cryptosporidium parvum infection. Infect. Immun. 66:4469–4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fayer, R., A. Guidry, and B. L. Blagburn. 1990. Immunotherapeutic efficacy of bovine colostral immunoglobulins from a hyperimmunized cow against cryptosporidiosis in neonatal mice. Infect. Immun. 58:2962–2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fayer, R., L. E. Perryman, and M. W. Riggs. 1989. Hyperimmune bovine colostrum neutralizes Cryptosporidium sporozoites and protects mice against oocyst challenge. J. Parasitol. 75:151–153. [PubMed] [Google Scholar]
- 30.Fayer, R., C. A. Speer, and J. P. Dubey. 1997. The general biology of Cryptosporidium, p.1–42. In R. Fayer (ed.), Cryptosporidium and cryptosporidiosis. CRC Press, Boca Raton, Fla.
- 31.Freedman, D. J., C. O. Tacket, A. Delehanty, D. R. Maneval, J. Nataro, and J. H. Crabb. 1998. Milk immunoglobulin with specific activity against purified colonization factor antigens can protect against oral challenge with enterotoxigenic Escherichia coli. J. Infect. Dis. 177:662–667. [DOI] [PubMed] [Google Scholar]
- 32.Griffiths, J. K., R. Balakrishnan, G. Widmer, and S. Tzipori. 1998. Paromomycin and geneticin inhibit intracellular Cryptosporidium parvum without trafficking through the host cell cytoplasm: implications for drug delivery. Infect. Immun. 66:3874–3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Griffiths, J. K., C. Theodos, M. Paris, and S. Tzipori. 1998. The gamma interferon gene knockout mouse: a highly sensitive model for evaluation of therapeutic agents against Cryptosporidium parvum. J. Clin. Microbiol. 36:2503–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamer, D. H., H. Ward, S. Tzipori, M. E. Pereira, J. P. Alroy, and G. T. Keusch. 1994. Attachment of Cryptosporidium parvum sporozoites to MDCK cells in vitro. Infect. Immun. 62:2208–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heine, J., J. F. L. Pohlenz, H. W. Moon, and G. N. Woode. 1984. Enteric lesions and diarrhea in gnotobiotic calves monoinfected with Cryptosporidium species. J. Infect. Dis. 150:768–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jenkins, M. C., C. O’Brien, J. Trout, A. Guidry, and R. Fayer. 1999. Hyperimmune bovine colostrum specific for recombinant Cryptosporidium parvum antigen confers partial protection against cryptosporidiosis in immunosuppressed adult mice. Vaccine 17:2453–2460. [DOI] [PubMed] [Google Scholar]
- 37.Joe, A., R. Verdon, S. Tzipori, G. T. Keusch, and H. D. Ward. 1998. Attachment of Cryptosporidium parvum sporozoites to human intestinal epithelial cells. Infect. Immun. 66:3429–3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lamm, M. E. 1997. Interaction of antigens and antibodies at mucosal surfaces. Annu. Rev. Microbiol. 51:311–340. [DOI] [PubMed] [Google Scholar]
- 39.Langer, R. C., and M. W. Riggs. 1999. Cryptosporidium parvum apical complex glycoprotein CSL contains a sporozoite ligand for intestinal epithelial cells. Infect. Immun. 67:5282–5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Langer, R. C., D. A. Schaefer, and M. W. Riggs. 2001. Characterization of an intestinal epithelial cell receptor recognized by the Cryptosporidium parvum sporozoite ligand CSL. Infect. Immun. 69:1661–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lindsay, D. S. 1997. Laboratory models of cryptosporidiosis, p.209–223. In R. Fayer (ed.), Cryptosporidium and cryptosporidiosis. CRC Press, Boca Raton, Fla.
- 42.Lumb, R., K. Smith, P. J. O’Donoghue, and J. A. Lanser. 1988. Ultrastructure of the attachment of Cryptosporidium sporozoites to tissue culture cells. Parasitol. Res. 74:531–536. [DOI] [PubMed] [Google Scholar]
- 43.Mazier, D., S. Mellouk, R. L. Beaudoin, B. Texier, P. Druilhe, W. Hockmeyer, J. Trosper, C. Paul, Y. Charoenvit, J. Young, F. Miltgen, L. Chedid, J. P. Chigot, B. Galley, O. Brandicourt, and M. Gentilini. 1986. Effect of antibodies to recombinant and synthetic peptides on P. falciparum sporozoites in vitro. Science 231:156–159. [DOI] [PubMed] [Google Scholar]
- 44.Mead, J. R., M. J. Arrowood, and C. R. Sterling. 1988. Antigens of Cryptosporidium sporozoites recognized by immune sera of infected animals and humans. J. Parasitol. 74:135–143. [PubMed] [Google Scholar]
- 45.Mineo, J. R., I. A. Kahn, and L. H. Kasper. 1994. Toxoplasma gondii: a monoclonal antibody that inhibits intracellular replication. Exp. Parasitol. 79:351–361. [DOI] [PubMed] [Google Scholar]
- 46.Naitza, S., F. Spano, K. J. H. Robson, and A. Crisanti. 1998. The thrombospondin-related protein family of apicomplexan parasites: the gears of the cell invasion machinery. Parasitol. Today 14:479–484. [DOI] [PubMed] [Google Scholar]
- 47.Nesterenko, M. V., K. Woods, and S. J. Upton. 1999. Receptor/ligand interactions between Cryptosporidium parvum and the surface of the host cell. Biochim. Biophys. Acta 1454:165–173. [DOI] [PubMed] [Google Scholar]
- 48.Nord, J., P. Ma, D. DiJohn, S. Tzipori, and C. O. Tacket. 1990. Treatment with bovine hyperimmune colostrum of cryptosporidial diarrhea in AIDS patients. AIDS 4:581–584. [DOI] [PubMed] [Google Scholar]
- 49.Nudelman, S., L. Renia, Y. Charoenvit, L. Yuan, F. Miltgen, R. L. Beaudoin, and D. Mazier. 1989. Dual action of anti-sporozoite antibodies in vitro. J. Immunol. 143:996–1000. [PubMed] [Google Scholar]
- 50.Okhuysen, P. C., C. L. Chappell, J. Crabb, L. M. Valdez, E. T. Douglass, and H. L. DuPont. 1998. Prophylactic effect of bovine anti-Cryptosporidium hyperimmune colostrum immunoglobulin in healthy volunteers challenged with Cryptosporidium parvum. Clin. Infect. Dis. 26:1324–1329. [DOI] [PubMed] [Google Scholar]
- 51.Perkins, M. E., Y. A. Riojas, T. W. Wu, and S. M. Le Blancq. 1999. CpABC, a Cryptosporidium parvum ATP-binding cassette protein at the host-parasite boundary in intracellular stages. Proc. Natl. Acad. Sci. USA 96:5734–5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perryman, L. E., D. P. Jasmer, M. W. Riggs, S. G. Bohnet, T. C. McGuire, and M. J. Arrowood. 1996. A cloned gene of Cryptosporidium parvum encodes neutralization-sensitive epitopes. Mol. Biochem. Parasitol. 80:137–147. [DOI] [PubMed] [Google Scholar]
- 53.Perryman, L. E., S. J. Kapil, M. L. Jones, and E. L. Hunt. 1999. Protection of calves against cryptosporidiosis with immune bovine colostrum induced by a Cryptosporidium parvum recombinant protein. Vaccine 17:2142–2149. [DOI] [PubMed] [Google Scholar]
- 54.Perryman, L. E., K. A. Kegerreis, and P. H. Mason. 1993. Effect of orally administered monoclonal antibody on persistent Cryptosporidium parvum infection in scid mice. Infect. Immun. 61:4906–4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perryman, L. E., M. W. Riggs, P. H. Mason, and R. Fayer. 1990. Kinetics of Cryptosporidium parvum sporozoite neutralization by monoclonal antibodies, immune bovine serum, and immune bovine colostrum. Infect. Immun. 58:257–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Petersen, C., J. Gut, P. S. Doyle, J. H. Crabb, R. G. Nelson, and J. H. Leech. 1992. Characterization of a >900,000-Mr Cryptosporidium parvum sporozoite glycoprotein recognized by protective hyperimmune bovine colostral immunoglobulin. Infect. Immun. 60:5132–5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Petry, F., and J. R. Harris. 1999. Ultrastructure, fractionation and biochemical analysis of Cryptosporidium parvum sporozoites. Int. J. Parasitol. 29:1249–1260. [DOI] [PubMed] [Google Scholar]
- 58.Priest, J. W., J. P. Kwon, M. J. Arrowood, and P. J. Lammie. 2000. Cloning of the immunodominant l7-kDa antigen from Cryptosporidium parvum. Mol. Biochem. Parasitol. 106:261–271. [DOI] [PubMed] [Google Scholar]
- 59.Riggs, M. W. 1997. Immunology: host response and development of passive immunotherapy and vaccines, p.129–161. In R. Fayer (ed.), Cryptosporidium and cryptosporidiosis. CRC Press, Boca Raton, Fla.
- 60.Riggs, M. W., V. A. Cama, H. L. Leary, Jr., and C. R. Sterling. 1994. Bovine antibody against Cryptosporidium parvum elicits a circumsporozoite precipitate-like reaction and has immunotherapeutic effect against persistent cryptosporidiosis in SCID mice. Infect. Immun. 62:1927–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Riggs, M. W., T. C. McGuire, P. H. Mason, and L. E. Perryman. 1989. Neutralization-sensitive epitopes are exposed on the surface of infectious Cryptosporidium parvum sporozoites. J. Immunol. 143:1340–1345. [PubMed] [Google Scholar]
- 62.Riggs, M. W., M. R. McNeil, L. E. Perryman, A. L. Stone, M. S. Scherman, and R. M. O’Connor. 1999. Cryptosporidium parvum sporozoite pellicle antigen recognized by a neutralizing monoclonal antibody is a β-mannosylated glycolipid. Infect. Immun. 67:1317–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Riggs, M. W., and L. E. Perryman. 1987. Infectivity and neutralization of Cryptosporidium parvum sporozoites. Infect. Immun. 55:2081–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Riggs, M. W., A. L. Stone, P. A. Yount, R. C. Langer, M. J. Arrowood, and D. L. Bentley. 1997. Protective monoclonal antibody defines a circumsporozoite-like glycoprotein exoantigen of Cryptosporidium parvum sporozoites and merozoites. J. Immunol. 158:1787–1795. [PubMed] [Google Scholar]
- 65.Roberts, F., C. W. Roberts, J. J. Johnson, D. E. Kyle, T. Krell, J. R. Coggins, G. H. Coombs, W. K. Milhous, S. Tzipori, D. J. P. Ferguson, D. Chakrabarti, and R. McLeod. 1998. Evidence for the shikimate pathway in apicomplexan parasites. Nature 393:801–805. [DOI] [PubMed] [Google Scholar]
- 66.Sagodira, S., D. Buzoni-Gatel, S. Iochmann, M. Naciri, and D. Bout. 1999. Protection of kids against Cryptosporidium parvum infection after immunization of dams with CP15-DNA. Vaccine 17:2346–2355. [DOI] [PubMed] [Google Scholar]
- 67.Schaefer, D. A., B. A. Auerbach-Dixon, and M. W. Riggs. 2000. Characterization and formulation of multiple epitope-specific neutralizing monoclonal antibodies for passive immunization against cryptosporidiosis. Infect. Immun. 68:2608–2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stewart, M. J., and J. P. Vanderberg. 1991. Malaria sporozoites release circumsporozoite protein from their apical end and translocate it along their surface. J. Protozool. 38:411–421. [DOI] [PubMed] [Google Scholar]
- 69.Strong, W. B., J. Gut, and R. G. Nelson. 2000. Cloning and sequence analysis of a highly polymorphic Cryptosporidium parvum gene encoding a 60-kilodalton glycoprotein and characterization of its 15- and 45-kilodalton zoite surface antigen products. Infect. Immun. 68:4117–4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tacket, C. O., S. B. Binion, E. Bostwick, G. Losonsky, M. J. Roy, and R. Edelman. 1992. Efficacy of bovine milk immunoglobulin concentrate in preventing illness after Shigella flexneri challenge. Am. J. Trop. Med. Hyg. 47:276–283. [DOI] [PubMed] [Google Scholar]
- 71.Tacket, C. O., G. Losonsky, H. Link, Y. Hoang, P. Guesry, H. Hilpert, and M. M. Levine. 1988. Protection by milk immunoglobulin concentrate against oral challenge with enterotoxigenic Escherichia coli. N. Engl. J. Med. 318:1240–1243. [DOI] [PubMed] [Google Scholar]
- 72.Theodos, C. M., J. K. Griffiths, J. D’Onfro, A. Fairfield, and S. Tzipori. 1998. Efficacy of nitazoxanide against Cryptosporidium parvum in cell culture and in animal models. Antimicrob. Agents Chemother. 42:1959–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Theodos, C. M., K. L. Sullivan, J. K. Griffiths, and S. Tzipori. 1997. Profiles of healing and nonhealing Cryptosporidium parvum infection in C57BL/6 mice with functional B and T lymphocytes: the extent of gamma interferon modulation determines the outcome of infection. Infect. Immun. 65:4761–4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tilley, M., S. J. Upton, R. Fayer, J. R. Barta, C. E. Chrisp, P. S. Freed, B. L. Blagburn, B. C. Anderson, and S. M. Barnard. 1991. Identification of a 15-kilodalton surface glycoprotein on sporozoites of Cryptosporidium parvum. Infect. Immun. 59:1002–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tzipori, S. 1998. Cryptosporidiosis: laboratory investigations and chemotherapy. Adv. Parasitol. 40:186–221. [DOI] [PubMed] [Google Scholar]
- 76.Tzipori, S., W. Rand, J. Griffiths, G. Widmer, and J. Crabb. 1994. Evaluation of an animal model system for cryptosporidiosis: therapeutic efficacy of paromomycin and hyperimmune bovine colostrum-immunoglobulin. Clin. Diagn. Lab. Immunol. 1:450–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tzipori, S., D. Roberton, D. A. Cooper, and L. White. 1987. Chronic cryptosporidial diarrhoea and hyperimmune cow colostrum. Lancet ii:344–345. [DOI] [PubMed] [Google Scholar]
- 78.Ungar, B. L. P., D. J. Ward, R. Fayer, and C. A. Quinn. 1990. Cessation of Cryptosporidium-associated diarrhea in an acquired immunodeficiency syndrome patient after treatment with hyperimmune bovine colostrum. Gastroenterology 98:486–489. [DOI] [PubMed] [Google Scholar]
- 79.Verdon, R., G. T. Keusch, S. Tzipori, S. A. Grubman, D. M. Jefferson, and H. D. Ward. 1997. An in vitro model of infection of human biliary epithelial cells by Cryptosporidium parvum. J. Infect. Dis. 175:1268–1272. [DOI] [PubMed] [Google Scholar]
- 80.Ward, H., and A. M. Cevallos. 1998. Cryptosporidium: molecular basis of host-parasite interaction. Adv. Parasitol. 40:151–185. [DOI] [PubMed] [Google Scholar]
- 81.Waters, W. R., B. Frydman, L. J. Marton, A. Valasinas, V. K. Reddy, J. A. Harp, M. J. Wannemuehler, and N. Yarlett. 2000. [1N,12N]bis(Ethyl)-cis-6,7-dehydrospermine: a new drug for treatment and prevention of Cryptosporidium parvum infection of mice deficient in T-cell receptor alpha. Antimicrob. Agents Chemother. 44:2891–2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zeitlin, L., R. A. Cone, T. R. Moench, and K. J. Whaley. 2000. Preventing infectious disease with passive immunization. Microbes Infect. 2:701–708. [DOI] [PubMed] [Google Scholar]
- 83.Zeitlin, L., R. A. Cone, and K. J. Whaley. 1999. Using monoclonal antibodies to prevent mucosal transmission of epidemic infectious diseases. Emerg. Infect. Dis. 5:54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]