Abstract
The correlation between uptake of moxifloxacin by THP-1, a continuous line of monocytic cells devoid of intrinsic bactericidal properties, and its activity against Staphylococcus aureus ATCC 25923, a susceptible reference strain (MIC and minimal bactericidal concentration of moxifloxacin, 0.1 mg/liter), was studied in a 5-h assay. The uptake of the drug, added to the culture medium at 0.2 to 32 mg/liter, was evaluated by high-performance liquid chromatography. The ratio of the cellular to extracellular concentration of moxifloxacin reached, at equilibrium, 4.36 ± 0.39 in uninfected cells and 6.25 ± 0.41 in infected cells. The intracellular activity of moxifloxacin, introduced into the extracellular fluid at 0.06 to 8 mg/liter, was determined by the enumeration of viable bacteria. At concentrations ≤0.2 mg/liter, the drug inhibited only the intracellular bacterial growth, while at concentrations ≥0.5 mg/liter, it decreased the bacterial inoculum by less than 1 log10 unit, with a maximum effect at 3 to 4 h, followed by regrowth of surviving bacteria to 80 to 120% of the original level at 5 h. In contrast, when killing curves were determined by using Mueller-Hinton broth with a similar inoculum (107 CFU/ml), moxifloxacin at concentrations ≥0.2 mg/liter reduced the inoculum by at least 3 log10 units at 3 to 4 h, leaving ≤0.1% survival at 24 h. Persisters exhibited a fluoroquinolone susceptibility identical to that of S. aureus ATCC 25923. Our data indicate that moxifloxacin at therapeutic extracellular concentrations accumulates approximately sixfold in infected THP-1 cells and remains active intracellularly, but significantly less active than under in vitro conditions.
Among bacteria pathogenic to humans, some are intracellular, either obligately, such as chlamydiae and rickettsia, or facultatively, such as Legionella pneumophila, Listeria monocytogenes, and mycobacteria, but most are extracellular (17, 28, 31). However, even typical extracellular pathogens, e.g., Staphylococcus aureus, may occasionally become intracellular (17, 28), at least within monocytes, macrophages, and polymorphonuclear leukocytes (PMNs) when host defense mechanisms are activated (31). Although phagocytosis is a very effective nonspecific defense mechanism, it may remain ineffective in certain circumstances, i.e., massive infestation or lack of bactericidal functions. Thus, intracellular accumulation is always an important criterion for the clinical effectiveness of an antibiotic. However, intracellular penetration is not to be equated with intracellular activity (16). Fluoroquinolones are claimed to have a high intracellular penetration and to be highly active against intracellular microorganisms. Nevertheless, although many studies were interested in either their cellular uptake (29) or their intracellular activity (1, 3, 19, 21, 30, 36), much less have investigated both phenomena (6, 9–11, 13, 14, 22, 23, 25–27, 37). Moreover, the last set of studies usually evaluated the uptake and intracellular activity of fluoroquinolones in PMNs, whose intrinsic bactericidal properties certainly interfere, and the single sampling times in short-term experiments provide no information on the bacterial killing rate (35). Finally, factors that may alter such evaluation, that is, the number of ingested bacteria per cell, the level of cell contamination by remaining extracellular bacteria, and the percentage of cell recovery, are rarely specified (13, 14, 25–27).
Moxifloxacin (BAY 12-8039) is a newly developed 8-methoxyquinolone with an improved activity against gram-positive cocci and anaerobes (2); a good penetration and/or bioactivity in tissues, fluids, and cells (2, 3, 19, 21, 25; H. Takemura, H. Yamamoto, H. Kunishima, T. Hara, H. Ikejima, S. Terakubo, K. Kanemitsu, and J. Shimada, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 2323, p. 191, 2000), and a low propensity for causing phototoxic reactions (2).
The purpose of this study was to develop a model suitable for correlating uptake and intracellular activity of antibiotics and to apply it to the new fluoroquinolone moxifloxacin. Thus, the penetration of moxifloxacin into human monocytic THP-1 cells, which are devoid of bactericidal functions, and its intracellular activity against S. aureus, a common bacterial pathogen widely used as test organism in analogous investigations (6, 9, 10, 13, 14, 25–27, 37), were studied at several sampling times in a 5-h assay. In addition, the intracellular activity of moxifloxacin was compared with its in vitro activity, as it is classically evaluated by microbiologists, i.e., by means of MICs, minimal bactericidal concentrations (MBCs), and killing curves. Finally, bacteria persisting after intracellular and extracellular exposure to moxifloxacin were examined to determine whether they were selected resistant mutants or not.
MATERIALS AND METHODS
Bacteria and cell line culture.
S. aureus ATCC 25923 was used to assess the activity of moxifloxacin. Bacterial cells persisting after moxifloxacin exposure in time-kill studies and in intracellular assays were collected. All these organisms were routinely cultured on Mueller-Hinton (MH) agar and broth (Sanofi Diagnostics Pasteur, Paris, France) at 37°C for 18 h. The THP-1 cell line is a continuous line of human monocytic cells (33). THP-1 cells were grown in RPMI 1640 medium (Sigma, Saint Quentin Fallavier, France), supplemented with 10% decomplemented (56°C, 30 min) fetal calf serum (Seromed-Biochrom, Berlin, Germany) at pH 7.4 in an atmosphere of 5% CO2 at 37°C. Cells were subcultured every fourth day at an initial density of 2 × 105 cells per ml.
Antibiotic susceptibility testing.
Standard powder of moxifloxacin (Bayer Pharma, Puteaux, France) was kindly provided by its manufacturer. MICs were determined by the broth dilution technique in standard conditions (final inoculum of 105 CFU per ml or per spot) (7). MBCs (per spot MBCs) were determined as recommended and defined as the lowest concentrations that killed 99.9% of the inoculum (7). Killing curves for moxifloxacin at different concentrations (0.06 to 8 mg/liter) were based on testing in MH broth using standard (105 CFU per ml) and nonstandard (107 CFU per ml) inocula (7). After 0, 1, 3, 4, 5, and 24 h of incubation, viable bacteria were enumerated by a conventional plating method: 100-μl aliquots were removed and serially diluted 10-fold in distilled water, and 100-μl portions of the appropriate dilutions were spread on MH agar plates. Colonies were counted after overnight incubation at 37°C.
Moxifloxacin uptake by THP-1 cells.
Uptake of moxifloxacin by THP-1 cells was determined by high-performance liquid chromatography (HPLC). Moxifloxacin was diluted in Dulbecco’s phosphate buffer and added at a final concentration of 0.2 to 32 mg/liter to a suspension of 4 × 106 THP-1 cells in RPMI 1640 medium. After different incubation times (3, 15, and 30 min and 1, 3, and 5 h) at 37°C, cells were simultaneously separated from the extracellular solution by means of differential centrifugation through a water-impermeable silicone-oil barrier (density = 1.021 g/cm3) and lysed into orthophosphoric acid (29). Cell pellets were washed twice with 200 μl of 1 M orthophosphoric acid, and supernatants were pooled. Samples (50 μl) of incubation media and cellular lysates were directly injected into the chromatograph, composed of two pumps (model HP1050; Hewlett-Packard Bios Analytic, Labège, France; Kontron model 420; Bio-Tek Instruments, Milan, Italy), an automatic injector (Hewlett-Packard Bios Analytic; model HP1050), a double-switching valve (Touzart et Matignon, Courtaboeuf, France), and a fluorescence detector (model SFM25; Bio-Tek, Saint-Quentin en Yvelines, France). Moxifloxacin was extracted “on line” by retention on a precolumn (Merck Clevenot, Fontenay-sous-Bois, France), using a mobile phase composed of 3% methanol in 10 mM K2HPO4, pH 5.4. A mixture of acetonitrile and 10 mM K2HPO4 (15/85 [vol/vol], pH 2.5) was used for elution and chromatographic separation on a Supelcosil ABZ analytical column (Supelco, Saint-Quentin Fallavier, France), with a flow rate of 1.25 ml/min. A backflush of the precolumn was performed after each elution to avoid clogging. Moxifloxacin was detected by fluorescence, using an excitation wavelength set at 296 nm, and a cutoff filter set at 485 nm. The standards were prepared extemporaneously by spiking cell lysates or incubation media with known amounts of moxifloxacin. The calibration curves were obtained by linear regression between the concentrations, and the areas of moxifloxacin peaks were determined by electronic integration. The calibration curves were linear up to concentrations of 1,000 ng/ml in 1 M orthoposphoric acid and 4,000 ng/ml in RPMI 1640 medium. Precision and accuracy of the HPLC assay were tested on 10 samples for three different concentrations; intraday and interday coefficient variations were lower than 4%. The lower limit of detection was 1 ng/ml. Intracellular concentrations of moxifloxacin were determined by dividing the amount of drug in the intracellular lysate by the volume of monocytes calculated according to the following formula: V = π/6 × D3 × y · 106, where D is the mean diameter and y · 106 is the number of cells estimated by microscopic examination. The uptake of moxifloxacin in THP-1 cells was expressed as the cellular-to-extracellular (C/E) ratio. Moxifloxacin uptake in THP-1 cells infected by S. aureus ATCC 25923 was also measured by the same procedure.
Intracellular activity of moxifloxacin.
After infection of THP-1 cells, moxifloxacin was added to the extracellular medium at eight concentrations ranging between 0.06 and 8 mg/liter, and intracellular viable bacteria were enumerated after incubation. Briefly, 2 ml of S. aureus ATCC 25923 suspension (108 CFU/ml), preopsonized in 40% pooled human serum, and 2 ml of THP-1 cells (106 cells/ml) were combined in a series of propylene biovials (Polylabo, Strasbourg, France). Extracellular bacteria were removed from the incubation medium after a 40-min incubation at 37°C by double differential centrifugation. First, the cell suspension was layered on a mixture composed of 50% phosphate-buffered saline (PBS), pH 7.2 and density of 1 g/cm3 (Biochrom KG, Berlin, Germany), and 50% lymphoprep, density 1.078 g/cm3 (Nycomed, Oslo, Norway), and was centrifuged at 14,000 × g for 5 min at 37°C. Then, the supernatant was discarded, and the remaining medium was diluted with PBS to decrease the density to 1.019 g/cm3. The suspension was centrifuged again (14,000 × g for 5 min at 37°C), and the pellet was resuspended in 2 ml of RPMI 1640. At this time (designated time zero), moxifloxacin was added at different final concentrations and the vials were reincubated at 37°C. Vials were removed after 0, 1, 3, 4, and 5 h of incubation (control without antimicrobial agent, and sample containing moxifloxacin at a defined concentration). Cells were pelleted (14,000 × g for 5 min at 37°C), washed in PBS, and then lysed in distilled water. Viable bacteria were enumerated by washing supernatants and cell lysates as indicated above. The number and the morphological aspect of the cells were checked by light microscopy examination at each step.
Statistical analysis.
All data concerning intracellular penetration of moxifloxacin were expressed as the means ± standard deviations of six independent tests; a statistical analysis of variance was performed by the Student t test with a P value ≤0.05 considered significant.
RESULTS
Antibiotic susceptibility of S. aureus ATCC 25923 and persisters.
S. aureus ATCC 25923 was susceptible to all 18 antibiotics tested by the disk diffusion method, and the persisters exhibited the same susceptibility profile. MICs and MBCs of moxifloxacin (Table 1) confirmed these data and demonstrated that moxifloxacin was bactericidal (MBC/MIC ratios of 1 to 2). Moxifloxacin exerted the same inhibitory and bactericidal effect on S. aureus ATCC 25923 and on the persisters.
TABLE 1.
Fluoroquinolone susceptibility of S. aureus ATCC 25923 and moxifloxacin persistersa
| Bacteria | Moxifloxacin
|
|
|---|---|---|
| MIC (mg/liter) | MBC (mg/liter) | |
| S. aureus ATCC 25923 | 0.1 | 0.1 |
| Persisters | ||
| Time-kill studies | ||
| Low inoculum (105 CFU/ml) | l0.1–0.2 | 0.1–0.2 |
| High inoculum (107 CFU/ml) | 0.1–0.2 | 0.1–0.2 |
| Intracellular assays | 0.1–0.2 | 0.1–0.2 |
The concentrations allowing growth of persisters ranged between 0.06 and 8 mg/liter for each experiment in time-kill studies and intracellular assays. All colonies of persisters were pooled for each concentration of moxifloxacin tested.
Time-kill studies.
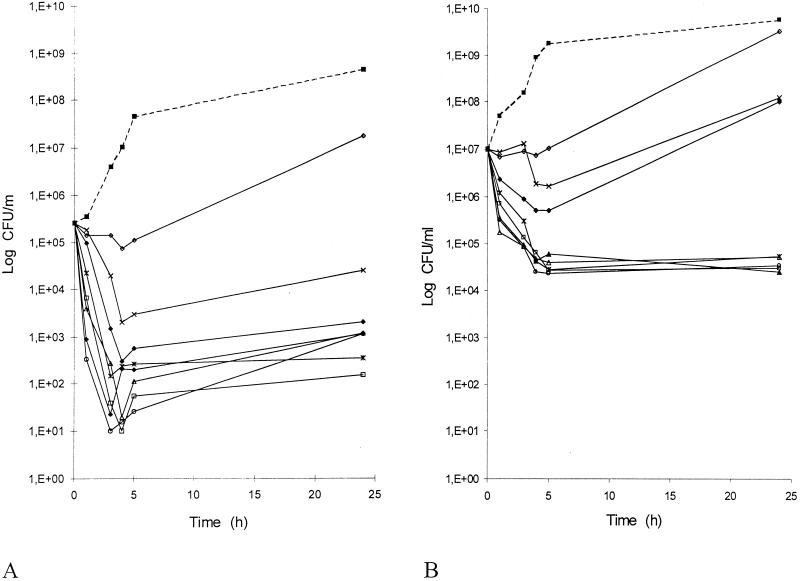
Killing curves for moxifloxacin against S. aureus ATCC 25923 were based on testing performed in MH broth at concentrations of 0.06 to 8 mg/liter, first using the recommended inoculum of 105 CFU/ml (7). Results (Fig. 1A) were consistent with MBC data. Indeed, moxifloxacin at 0.06 and 0.1 mg/liter only inhibited the bacterial growth. The drug was bactericidal (reduction of the bacterial inoculum by at least 3 log10 units after a 24-h incubation) at concentrations equal to or greater than 0.2 mg/liter. These concentrations (0.2 to 8 mg/liter) led to highly similar curves. There was a rapid initial decrease in viable bacteria, with a maximum effect (reduction of 3 to 4 log10 units) after 3 to 5 h of incubation, followed by a bacterial regrowth leaving persistent cells at 24 h (less than 0.1% of the inoculum at concentrations ≥0.2 mg/liter).
FIG. 1.
Influence of moxifloxacin on the viability of S. aureus ATCC 25923 using inocula of 105 (A) and 107 CFU/ml (B). The antibacterial effect of moxifloxacin was expressed as the mean number of viable bacteria obtained in two determinations. This was normalized for the control growth curve to allow comparison between antibiotic concentrations. Symbols: ▪, control; ◊, 0.06 mg/liter; ×, 0.1 mg/liter ⧫, 0.2 mg/liter; *, 0.5 mg/liter; □, 1 mg/liter; ○, 2 mg/liter; ▴, 4 mg/liter; ▵, 8 mg/liter.
Then, the same experiments were done using a higher inoculum (107 CFU/ml), equivalent to the number of organisms per milliliter of cells. There was a slight inoculum size effect (Fig. 1B). Indeed, a bactericidal activity was observed for slightly higher concentrations (≥0.5 mg/liter), which gave analogous curves. The maximal effect was slightly less marked (reduction of the inoculum by 2.5 to 3 log10 units) and delayed (at 4 to 5 h) and was followed by bacterial persistence (less than 0.1% of the inoculum at concentrations ≥0.5 mg/liter).
Uptake of moxifloxacin in THP-1 cells.
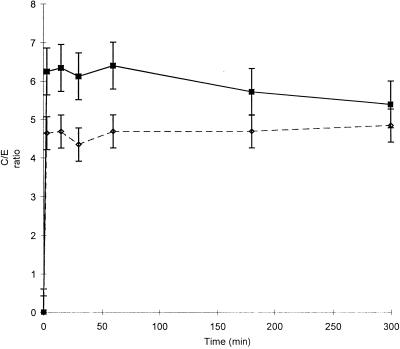
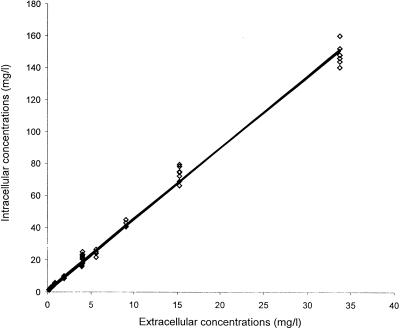
Penetration and accumulation of moxifloxacin in THP-1 cells were evaluated under basal conditions (uninfected cells, 37°C, 5% CO2, pH 7.35), with 11 extracellular concentrations ranging from 0.2 to 32 mg/liter, at seven sampling times in a 5-h assay (Fig. 2). Moxifloxacin penetrated very rapidly into THP-1 cells, since uptake was essentially complete after only 5 min of incubation with the drug. At equilibrium, cellular concentrations of moxifloxacin were four to five times greater than the extracellular ones (C/E ratio: 4.36 ± 0.39). They tended to slightly decrease over the 5-h period of incubation, but the variations observed were not statistically significant. In uninfected cells, there was a close correlation (r2 = 0.993, n = 80) between intra- and extracellular concentrations of moxifloxacin (Fig. 3). However, analysis of variance demonstrated that the mean C/E ratio varied according to extracellular concentrations. C/E ratios were significantly higher for extracellular concentrations ≤0.5 μg/ml than for concentrations greater than this value (C/E ratios: 7.11 ± 0.62 for 0.3 μg/ml and 7.22 ± 0.55 for 0.5 μg/ml versus 4.81 ± 0.29 for 16 μg/ml; P < 0.05). The presence of ingested S. aureus significantly increased (P < 0.01) the intracellular penetration by moxifloxacin of THP-1 cells during the first hour of incubation. At equilibrium, the C/E ratios in infected cells reached 6.25 ± 0.41 (Fig. 2).
FIG. 2.
Time profiles of moxifloxacin uptake by THP-1 cells infected by S. aureus (▪) or uninfected (◊) at an extracellular concentration of 4 mg/liter for 300 min. C/E ratios represent the means of six experiments. Data were compared by the Student t test.
FIG. 3.
Moxifloxacin uptake by uninfected THP-1 cells with different extracellular concentrations. The uptake values represent six experiments for each tested concentration.
Finalization of the model.
In preliminary experiments, several methods of bacterial infestation and elimination of extracellular bacteria were investigated. Higher cell densities or longer incubation times led to cellular death. Opsonization by fetal calf serum and human plasma or pig serum was less efficient for bacterial infestation (0.01 and 0.025 CFU/cell, respectively). A bacterium/cell ratio of 1,000 impaired the elimination of extracellular bacteria (contamination of 13.8%). Removal of noninternalized bacteria by simple washings left 14% of contaminating extracellular bacteria. Treatment with gentamicin (20 mg/liter for 2 h) yielded 69% cellular death (12). Lysostaphin (30 mg/liter for 10 min) gave satisfactory results (0.05% of contaminating bacteria left). However, this reagent was not used further, since it is only useful for staphylococcal elimination, and, like gentamicin, it has been reported to penetrate into cells (15, 16, 28, 34, 35). The incubation time was not extended beyond 5 h because, in the control, cells began to lyse and bacteria began to become extracellular. Our model provided an infestation rate of 0.34 CFU per cell, and 90% of the cells were recovered. The level of cell contamination was about 1% of the final bacterial count, as determined by bacterial enumeration after the final washing in both the supernatant and the lysate of the cell pellet.
Intracellular activity of moxifloxacin.
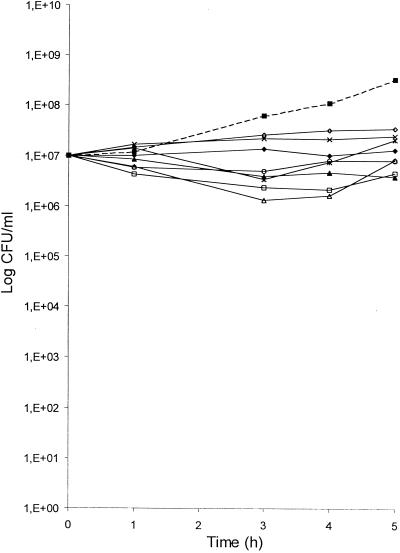
The kinetics of the intracellular activity of moxifloxacin against S. aureus ATCC 25923 in THP-1 cells was determined at eight extracellular concentrations equivalent to clinical serum levels (0.06 to 8 mg/liter) and at four sampling times (1, 3, 4, and 5 h) over a 5-h period of incubation (Fig. 4). When infested cells were incubated in the absence of the antibiotic, there was a substantial bacterial growth since the number of viable S. aureus cells after 5 h increased by 1.5 log10 units compared with the number at time zero. However, THP-1 cells demonstrated a slight inhibitory activity since the intracellular growth was reduced by 1 log10 CFU/ml compared with that from curves based on growth in MH broth with either a low or a high inoculum. The addition of moxifloxacin to the extracellular medium resulted in a concentration- and time-dependent decrease of the number of cell-associated S. aureus cells. At final concentrations ≤0.2 mg/liter in the extracellular fluid, moxifloxacin only inhibited the bacterial multiplication, leading to bacterial counts similar to the time zero value (inoculum). This bacteriostatic effect corresponded to percentages of surviving staphylococci compared with percentages for control cells (without antibiotic) taken at the same sampling times of 55 versus 21%, respectively, at 3 h and 10 versus 4%, respectively, at 5 h. At extracellular concentrations ≥0.5 mg/l, there was a transient and moderate reduction of the inoculum. The highest effect was seen at 1 to 4 h, reaching a 1 log10 unit reduction of the inoculum. Again, it was followed by regrowth leading to the presence of approximately 80% of the inoculum at 5 h. This moderate bactericidal effect corresponded to percentages of surviving staphylococci compared with percentages for control cells taken at the same times of 8 versus 2%, respectively, at 3 h and of 6 versus 1%, respectively, at 5 h. Extracellular concentrations ≥0.5 mg/liter led to almost identical curves. Thus moxifloxacin did not display a bactericidal activity, as microbiologically defined, against intracellular S. aureus, even at concentrations that were bactericidal for extracellular bacteria despite an accumulation in THP-1 cells at a level five- to sixfold greater than that in the extracellular medium. Actually, the calculated intracellular concentrations (CIC) of moxifloxacin, on a C/E ratio basis of 6 (0.4 to 50 mg/liter), always largely exceeded the MBC of the drug for S. aureus ATCC 25923 (approximately 4 to 500 times the MBC).
FIG. 4.
Effect of moxifloxacin on the viability of S. aureus ATCC 25923 ingested by THP-1 cells. Antibacterial effect of moxifloxacin was expressed as the mean number of viable bacteria obtained in three determinations. This was normalized for the control growth curve to allow comparison between antibiotic concentrations. Symbols: ▪, control; ◊, 0.06 mg/liter, ×, 0.1 mg/liter; ⧫, 0.2 mg/liter; *, 0.5 mg/liter; □, 1 mg/liter; ○, 2 mg/liter; ▴, 4 mg/liter; ▵, 8 mg/liter.
DISCUSSION
In this study, we developed a sensitive model of S. aureus-infected human monocytes for analyzing uptake and intracellular activity of antibiotics. Monocytes are, with PMNs, one of the two types of phagocytic white blood cells that are the primary line of cellular defense against microbial infection (31). THP-1 cells are capable of phagocytosis (33) but do not kill intracellular bacteria (30), minimizing direct and indirect interactions between antibiotics and phagocyte functions (15). S. aureus is an extracellular pathogen which may, occasionally, survive and even multiply within phagocytes, resulting in prolonged and recurrent infections (17, 28). Probably because S. aureus does not promote its own internalization, cell infestation required a higher cellular density, a prolonged opsonization, and a more elevated bacterium-to-cell ratio than L. monocytogenes (30). Almost complete removal of noninternalized bacteria and cell recovery were achieved by two differential centrifugations, in contrast with other methods (6, 9–11, 19, 21, 30, 37), which may induce overestimation of the intracellular activity of antibiotics (35).
The uptake of moxifloxacin by THP-1 cells was evaluated by HPLC. Methods used for measuring fluoroquinolone uptake have provided very similar results (35). Penetration of moxifloxacin into THP-1 cells proceeded very rapidly, as previously observed in THP-1 cells (Takemura et al., 40th ICAAC) and PMNs (25). At steady state, moxifloxacin gave intracellular concentrations four to five times higher (4.36 ± 0.39) than the extracellular ones, i.e., slightly less than that observed previously (Takemura et al., 40th ICAAC) (6.3 ± 0.9). Pascual et al. (25) reported C/E ratios for moxifloxacin in PMNs and McCoy cells of ca. 11 and 9, respectively. Fluoroquinolones usually reach a plateau within few minutes of incubation, at C/E ratios ranging between 2 and 10 (6, 9, 10, 13, 15, 23–26, 29, 37), in phagocytic cells and at lower levels in nonphagocytic cells (6, 13, 24–26), although Takemura et al. (40th ICAAC) found a value of ca. 37 for moxifloxacin in alveolar epithelial cells. The mechanisms whereby quinolones accumulate in cells are not yet known. Passive diffusion is probably the major mechanism, but active transport systems are certainly involved (24, 25), which explains why higher C/E ratios can be observed at the lowest extracellular concentrations. S. aureus phagocytosis significantly enhanced the entry of moxifloxacin into THP-1 cells; this finding contrasts with previous reports on this molecule (25) and other quinolones (9, 10, 13, 14, 26, 27) in PMNs. However, variable effects of S. aureus ingestion on antibiotic uptake have been previously described (16). Indeed, phagocytosis of bacteria induces important changes in cells which are likely to affect the intracellular penetration of antibiotics, maybe depending on the cell type and the drug.
The intracellular activity against S. aureus ATCC 25923 of moxifloxacin in THP-1 cells was determined at therapeutic extracellular concentrations (0.06 to 8 mg/liter), in a 5-h assay. The intracellular activity of the antibiotic appeared to be concentration and time dependent. At concentrations ≤0.2 mg/liter (CIC: 4 to 12 × MBC), moxifloxacin exerted only a bacteriostatic effect. At levels ≥0.5 mg/liter (CIC: 40 to 500 × MBC), moxifloxacin displayed a modest intracellular bactericidal effect, since the antibiotic transiently reduced the inoculum by at most 1 log10 unit at 3 to 4 h. Beyond that time, regrowth up to ca. 80% of the inoculum value occurred. Thus, moxifloxacin remained active intracellularly and inhibited cell-associated S. aureus growth but to a much lesser extent than expected in view of its C/E ratios in THP-1 cells and its bactericidal activity against the test organism. Such data are in contradiction with the literature. Indeed, Pascual et al. (25) have reported a significant activity of moxifloxacin against S. aureus ATCC 25923 in PMNs. However, at external concentrations of 0.1 to 5 mg/liter (CIC: 20 to 1,000 × MBC), the antibiotic only gave, at the single sampling time of 3 h, 90% survival relative to 15% for control cells. Similar studies of other fluoroquinolones (10, 11, 13, 14, 26, 27, 37) have led to similar results and interpretation. Indeed, fluoroquinolones certainly cooperate with PMNs for killing bacteria (36), but the very efficient bactericidal mechanisms of these phagocytes render the PMN models poorly sensitive for evaluating the intrinsic cell bactericidal activity of antibiotics (35). Other studies only dealing with the intracellular bioactivity of moxifloxacin have demonstrated a bacteriostatic rather than a bactericidal effect (3, 19, 21; Takemura et al., 40th ICAAC). Similar investigations of the activity of other fluoroquinolones against nonstaphylococcal pathogens generally showed uncomplete eradication despite relatively high extracellular concentrations (11, 22, 23). Thus, the discrepancy between our data and those of other investigators might be related to the use of different cell types (microbicidal versus nonmicrobicidal cells) and expression of results (relative versus absolute data).
To further characterize the disparity between the intra- and extracellular activities of moxifloxacin against S. aureus ATCC 25923, the latter was reexamined. The MIC and MBC of moxifloxacin were identical to those determined by other investigators for this strain (25). Killing curves generated under standard conditions (inoculum of 105 CFU/ml) were consistent with these data and showed a concentration- and time-dependent bactericidal effect, in contrast with some previous pharmacodynamic studies of moxifloxacin (4, 5, 8, 20) but in agreement with others (2) and with the literature on the bactericidal activity of quinolones. Indeed, quinolones are known to produce concentration-dependent killing to a point of maximum effect. Higher concentrations do not increase either the killing rate or the total number of bacteria killed (18). Killing curves generated with a higher inoculum (107 CFU/ml), equivalent to the number of bacteria per milliliter of cell content, were not significantly different: increasing the inoculum size has little or no effect on the activity of moxifloxacin (2).
Contrasts between antibiotic uptake and subsequent intracellular bactericidal activity have been mentioned for various antimicrobial agents (16, 34, 35). The most probable explanation for the relative lack of antistaphylococcal activity of moxifloxacin in THP-1 cells is different subcellular distributions of the antibiotic and the microorganism. Indeed, fluoroquinolones seem largely if not exclusively located in the cytosol (28, 35), whereas S. aureus is believed to be sequestered in phagolysosomes (17, 28, 32, 34). Moreover, quinolones should be, at least partly, inactivated at the low pH of phagosomes (32). Partial binding of the drug to cell components is unlikely, since moxifloxacin (25), like other quinolones (11, 13, 14, 23, 26, 27, 34, 37) effluxes rapidly when the extracellular drug is removed. Given the potential of quinolones for emergence of resistance, persisters were examined to establish whether they were selected resistant mutants. According to MIC and MBC data, they were obviously not. There is always a small percentage of bacteria that are not completely eliminated by quinolone treatment. The molecular basis of the persister state remains mysterious (18).
In conclusion, the use of this model offers an interesting insight into the correlation between uptake and intracellular activity of antibiotics. When the model was applied to moxifloxacin, this fluoroquinolone was found to accumulate in infected THP-1 cells at a C/E ratio of approximately 6 and to remain active within these cells, but significantly less active than under in vitro conditions. In particular, moxifloxacin was not bactericidal in the accepted microbiological definition of this term. It would be of interest to adapt this model to other fluoroquinolones and microorganisms since pathogens, like antibiotics, may reside in different compartments of the cells. Maturation to macrophages and subsequent activation by lymphokines would allow further intracellular pharmacological and pharmacodynamic studies of antibiotics in the mononuclear phagocyte system.
Acknowledgments
We thank Catherine Andre for expert technical assistance.
This work was supported by a grant from the Ministère de l’Education Nationale et de la Recherche (EA-525), Université de Bordeaux 2.
REFERENCES
- 1.Al-Nawas, B., and P. M. Shah. 1998. Intracellular activity of ciprofloxacin and moxifloxacin, a new 8-methoxyquinolone, against methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 41:655–658. [DOI] [PubMed] [Google Scholar]
- 2.Balfour, J. A., and H. M. Lamb. 2000. Moxifloxacin: a review of its clinical potential in the management of community-acquired respiratory tract infections. Drugs 59:115–139. [DOI] [PubMed] [Google Scholar]
- 3.Bermudez, L. E., C. B. Inderlied, P. Kolonoski, M. Petrofsky, P. Aralar, M. Wu, and L. S. Young. 2001. Activity of moxifloxacin by itself and in combination with ethambutol, rifabutin, and azithromycin in vitro and in vivo against Mycobacterium avium. Antimicrob. Agents Chemother. 45:217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boswell, F. J., J. M. Andrews, and R. Wise. 1997. Pharmacodynamic properties of BAY 12-8039 on gram-positive and gram-negative organisms as demonstrated by studies of time-kill kinetics and postantibiotic effect. Antimicrob. Agents Chemother. 41:1377–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boswell, F. J., J. M. Andrews, R. Wise, and A. Dalhoff. 1999. Bactericidal properties of moxifloxacin and post-antibiotic effect. J. Antimicrob. Chemother. 43(Suppl. B):43–49. [DOI] [PubMed] [Google Scholar]
- 6.Carlier, M. B., B. Scorneaux, A. Zenebergh, J. F. Desnottes, and P. M. Tulkens. 1990. Cellular uptake, localization and activity of fluoroquinolones in uninfected and infected macrophages. J. Antimicrob. Chemother. 26(Suppl. B):27–39. [DOI] [PubMed] [Google Scholar]
- 7.Courvalin, P., F. Goldstein, A. Philippon, and J. Sirot. 1985. L’antibiogramme. MPC-Vidéom, Paris, France.
- 8.Dalhoff, A. 1999. Pharmacodynamics of fluoroquinolones. J. Antimicrob. Chemother. 43(Suppl. B):51–59. [DOI] [PubMed] [Google Scholar]
- 9.Easmon, C. S., and J. P. Crane. 1985. Uptake of ciprofloxacin by macrophages. J. Clin. Pathol. 38:442–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Easmon, C. S., J. P. Crane, and A. Blowers. 1986. Effect of ciprofloxacin on intracellular organisms: in-vitro and in-vivo studies. J. Antimicrob. Chemother. 18(Suppl. D):43–48. [DOI] [PubMed] [Google Scholar]
- 11.Edelstein, P. H., M. A. Edelstein, J. Ren, R. Polzer, and R. P. Gladue. 1996. Activity of trovafloxacin (CP-99,219) against Legionella isolates: in vitro activity, intracellular accumulation and killing in macrophages, and pharmacokinetics and treatment of guinea pigs with L. pneumophila pneumonia. Antimicrob. Agents Chemother. 40:314–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Facinelli, B., G. Magi, M. Prenna, S. Ripa, and P. E. Varaldo. 1997. In vitro extracellular and intracellular activity of two newer and two earlier fluoroquinolones against Listeria monocytogenes. Eur. J. Clin. Microbiol. Infect. Dis. 16:827–833. [DOI] [PubMed] [Google Scholar]
- 13.Garcia, I., A. Pascual, M. C. Guzman, and E. J. Perea. 1992. Uptake and intracellular activity of sparfloxacin in human polymorphonuclear leukocytes and tissue culture cells. Antimicrob. Agents Chemother. 36:1053–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia, I., A. Pascual, and E. J. Perea. 1994. Intracellular penetration and activity of BAY Y 3118 in human polymorphonuclear leukocytes. Antimicrob. Agents Chemother. 38:2426–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gemmell, C. G. 1993. Antibiotics and neutrophils function—potential immunomodulating activities. J. Antimicrob. Chemother. 31(Suppl. B):23–33. [DOI] [PubMed] [Google Scholar]
- 16.Hand, W. L., and N. L. King-Thompson. 1986. Contrasts between phagocyte antibiotic uptake and subsequent intracellular bactericidal activity. Antimicrob. Agents Chemother. 29:135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hof, H. 1991. Intracellular microorganisms: a particular problem for chemotherapy. Infection 19(Suppl. 4):S193–S194. [DOI] [PubMed] [Google Scholar]
- 18.Hooper, D. C., and J. S. Wolfson. 1993. Mechanisms of quinolone action and bacterial killing, p.53–75. In D. C. Hooper and J. S. Wolfson (ed.), Quinolone antimicrobial agents, 2nd ed. American Society for Microbiology, Washington, D.C.
- 19.Jonas, D., I. Engels, C. Friedhoff, B. Spitzmuller, F. D. Daschner, and U. Frank. 2001. Efficacy of moxifloxacin, trovafloxacin, clinafloxacin and levofloxacin against intracellular Legionella pneumophila. J. Antimicrob. Chemother. 47:147–152. [DOI] [PubMed] [Google Scholar]
- 20.MacGowan, A. P., K. E. Bowker, M. Wootton, and H. A. Holt. 1999. Exploration of the in-vitro pharmacodynamic activity of moxifloxacin for Staphylococcus aureus and streptococci of Lancefield groups A and G. J. Antimicrob. Chemother. 44:761–766. [DOI] [PubMed] [Google Scholar]
- 21.Mandell, G. L., and E. J. Coleman. 2000. Activities of antimicrobial agents against intracellular pneumococci. Antimicrob. Agents Chemother. 44:2561–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mor, N., J. Vanderkolk, and L. Heifets. 1994. Inhibitory and bactericidal activities of levofloxacin against Mycobacterium tuberculosis in vitro and in human macrophages. Antimicrob. Agents Chemother. 38:1161–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ozaki, M., K. Komori, M. Matsuda, R. Yamaguchi, T. Honmura, Y. Tomii, I. Nishimura, and T. Nishino. 1996. Uptake and intracellular activity of NM394, a new quinolone, in human polymorphonuclear leukocytes. Antimicrob. Agents Chemother. 40:739–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pascual, A. 1995. Uptake and intracellular activity of antimicrobial agents in phagocytic cells. Rev. Med. Microbiol. 6:228–235. [Google Scholar]
- 25.Pascual, A., I. Garcia, S. Ballesta, and E. J. Perea. 1999. Uptake and intracellular activity of moxifloxacin in human neutrophils and tissue-cultured epithelial cells. Antimicrob. Agents Chemother. 43:12–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pascual, A., I. Garcia, S. Ballesta, and E. J. Perea. 1997. Uptake and intracellular activity of trovafloxacin in human phagocytes and tissue-cultured epithelial cells. Antimicrob. Agents Chemother. 41:274–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pascual, A., I. Garcia, and E. J. Perea. 1990. Uptake and intracellular activity of an optically active ofloxacin isomer in human neutrophils and tissue culture cells. Antimicrob. Agents Chemother. 34:277–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rakita, R. M. 1998. Intracellular activity, potential clinical uses of antibiotic. ASM News 64:570–575. [Google Scholar]
- 29.Rispal, P., J. Grellet, C. Celerier, D. Breilh, M. Dorian, J. L. Pellegrin, M. C. Saux, and B. Leng. 1996. Comparative uptake of sparfloxacin and ciprofloxacin into human THP 1 monocytic cells. Arzneim.-Forsch. 46:316–319. [PubMed] [Google Scholar]
- 30.Scorneaux, B., Y. Ouadrhiri, G. Anzalone, and P. M. Tulkens. 1996. Effect of recombinant human gamma interferon on intracellular activities of antibiotics against Listeria monocytogenes in the human macrophage cell line THP-1. Antimicrob. Agents Chemother. 40:1225–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silverstein, S. C., and T. H. Steinberg. 1990. Host defense against bacterial and fungal infections, p.485–505. In B. D. Davis, R. Dulbecco, H. N. Eisen, and H. S. Ginsberg (ed.), J. P. Lippincott Company, Philadelphia, Pa.
- 32.Steinberg, T. H. 1994. Cellular transport of drugs. Clin. Infect. Dis. 19:916–921. [DOI] [PubMed] [Google Scholar]
- 33.Tsuchiya, S., M. Yamabe, Y. Yamaguchi, Y. Kobayashi, T. Konno, and K. Tada. 1980. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int. J. Cancer 26:171–176. [DOI] [PubMed] [Google Scholar]
- 34.Tulkens, P. M. 1991. Intracellular pharmacokinetics and localization of antibiotics as predictors of their efficacy against intraphagocytic infections. Scand. J. Infect. Dis. (Suppl. 74) :209–217. [PubMed]
- 35.Van der Auwera, P. 1990. In-vitro tests of the functions of phagocytic cells and their interaction with antimicrobial agents: a critical view. J. Antimicrob. Chemother. 26:168–173. [DOI] [PubMed] [Google Scholar]
- 36.Van Rensburg, C. E., G. Joone, and R. Anderson. 1989. An in vitro investigation of the intraphagocytic bioactivity of difloxacin, ciprofloxacin, pefloxacin and fleroxacin. Chemotherapy 35:273–277. [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto, T., H. Kusajima, M. Hosaka, and H. Shinoda. 1995. Uptake and intracellular activity of fleroxacin in phagocytic cells. Chemotherapy 41:353–359. [DOI] [PubMed] [Google Scholar]