Abstract
1. The activities of fructose 1,6-diphosphatase were measured in extracts of muscles of various physiological function, and compared with the activities of other enzymes including phosphofructokinase, phosphoenolpyruvate carboxykinase and the lactate-dehydrogenase isoenzymes. 2. The activity of phosphofructokinase greatly exceeded that of fructose diphosphatase in all muscles tested, and it is concluded that fructose diphosphatase could not play any significant role in the regulation of fructose 6-phosphate phosphorylation in muscle. 3. Fructose-diphosphatase activity was highest in white muscle and low in red muscle. No activity was detected in heart or a deep-red skeletal muscle, rabbit semitendinosus. 4. The lactate-dehydrogenase isoenzyme ratio (activities at high and low substrate concentration) was measured in various muscles because a low ratio is characteristic of muscles that are more dependent on glycolysis for their energy production. As the ratio decreased the activity of fructose diphosphatase increased, which suggests that highest fructose-diphosphatase activity is found in muscles that depend most on glycolysis. 5. There was a good correlation between the activities of fructose diphosphatase and phosphoenolpyruvate carboxykinase in white muscle, where the activities of these enzymes were similar to those of liver and kidney cortex. However, the activities of pyruvate carboxylase and glucose 6-phosphatase were very low in white muscle, thereby excluding the possibility of gluconeogenesis from pyruvate and lactate. 6. It is suggested that the presence of fructose diphosphatase and phosphoenolpyruvate carboxykinase in white muscle may be related to operation of the α-glycerophosphate–dihydroxyacetone phosphate and malate–oxaloacetate cycles in this tissue.
Full text
PDF

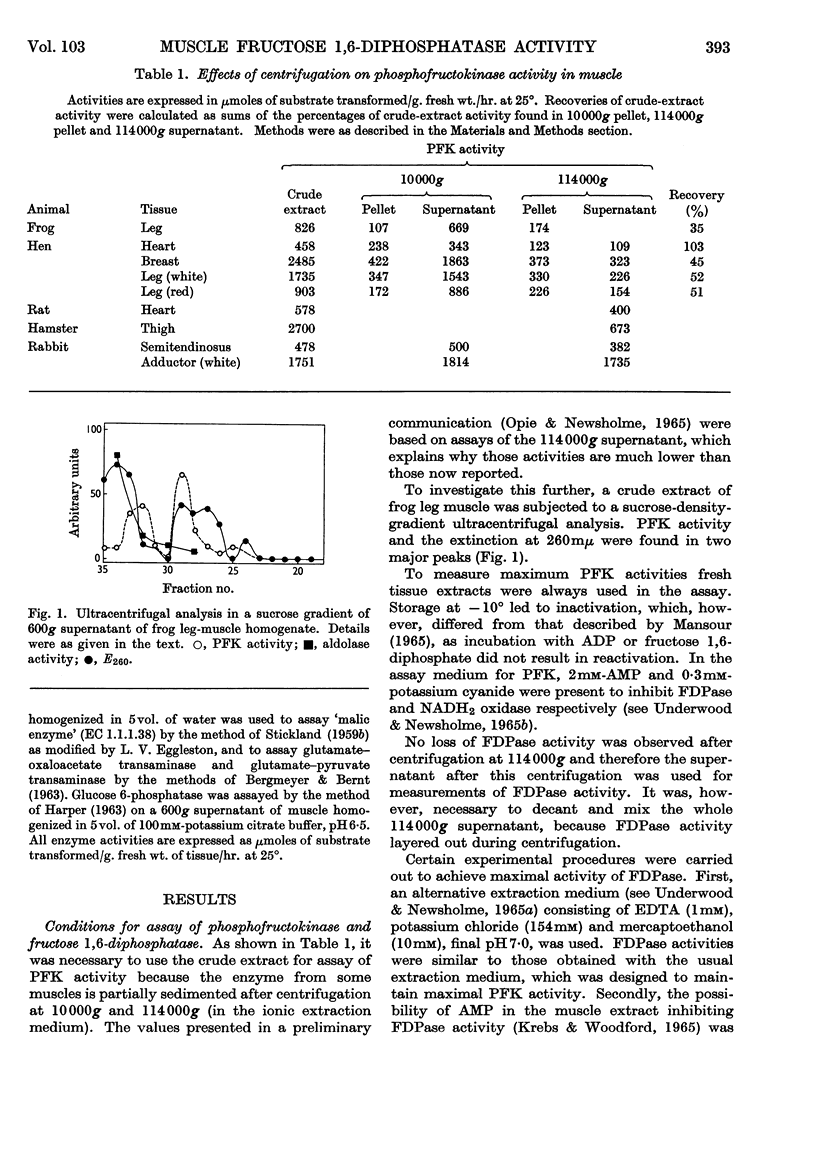

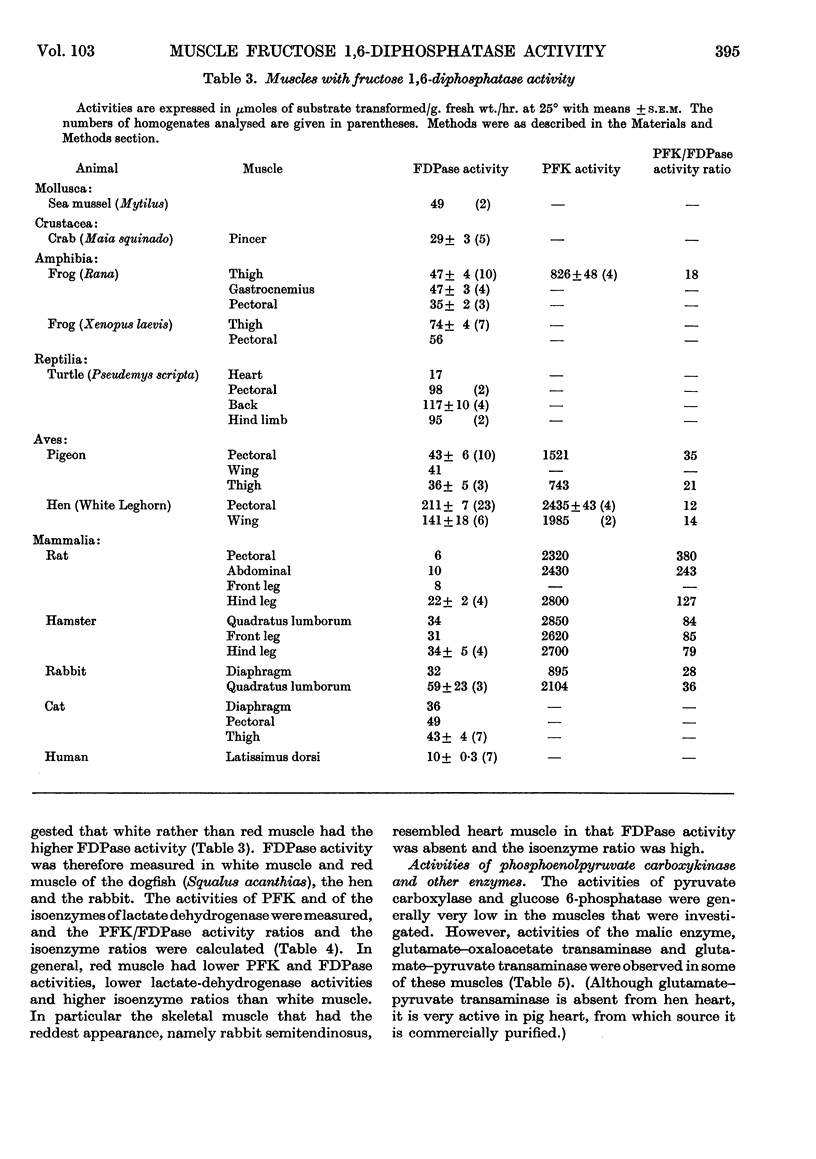
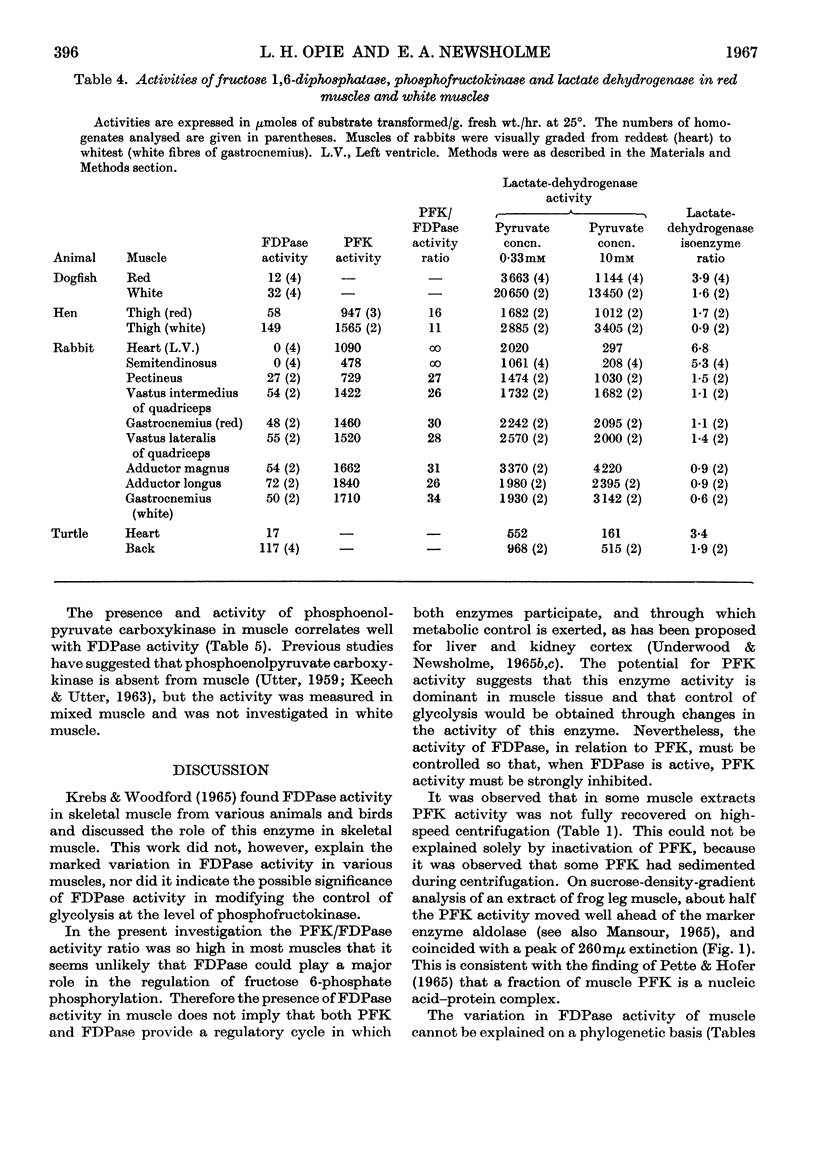
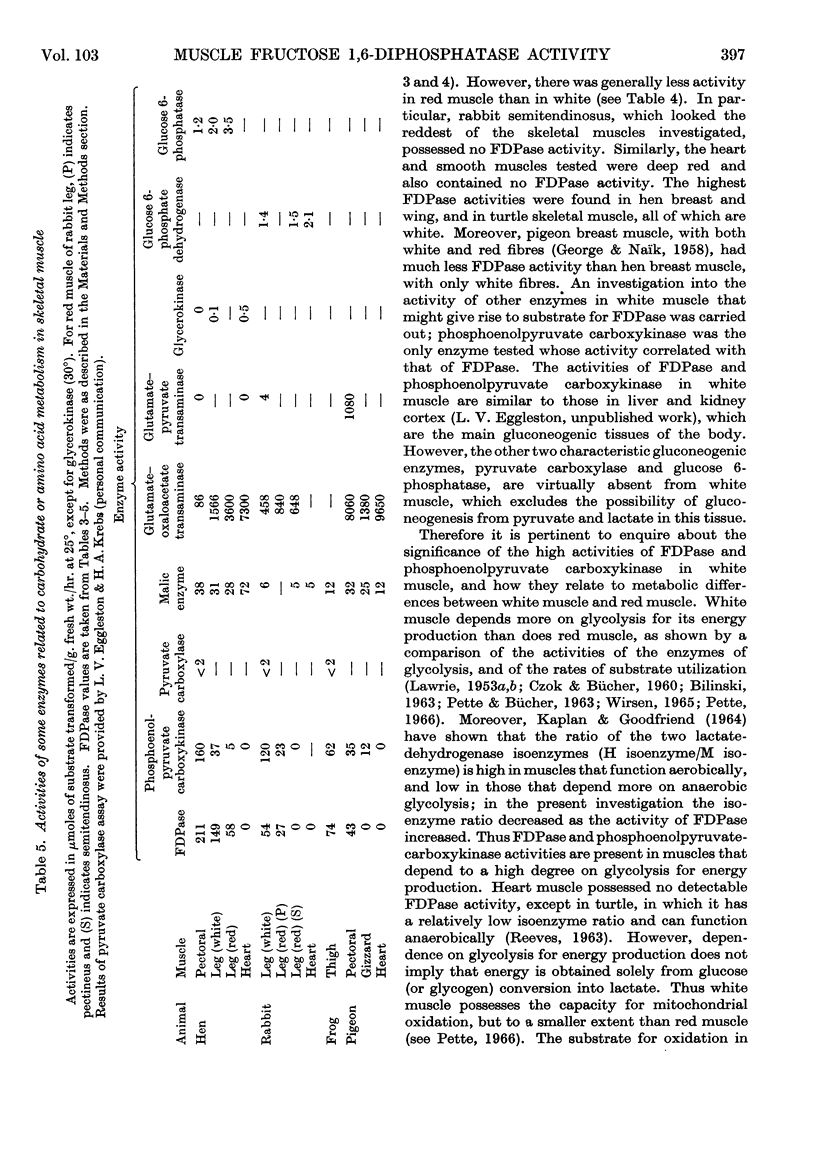


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BILINSKI E. Utilizatimn lipids by fish. I. Fatty acid oxidation by tissue slices from dark and white muscle of rainbow trout (Salmo gairdnerii). Can J Biochem Physiol. 1963 Jan;41:107–112. [PubMed] [Google Scholar]
- BLANCHAER M. C. RESPIRATION OF MITOCHONDRIA OF RED AND WHITE SKELETAL MUSCLE. Am J Physiol. 1964 May;206:1015–1020. doi: 10.1152/ajplegacy.1964.206.5.1015. [DOI] [PubMed] [Google Scholar]
- Boyland E., Sims P. The metabolism of benz[a]anthracene and dibenz[a,h]anthracene and their 5,6-epoxy-5,6-dihydro derivatives by rat-liver homogenates. Biochem J. 1965 Oct;97(1):7–16. doi: 10.1042/bj0970007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CZOK R., BUECHER T. Crystallized enzymes from the myogen of rabbit skeletal muscle. Adv Protein Chem. 1960;15:315–415. doi: 10.1016/s0065-3233(08)60311-3. [DOI] [PubMed] [Google Scholar]
- GEORGE J. C., NAIK R. M. Relative distribution and chemical nature of the fuel store of the two types of fibres in the pectoralis major muscle of the pigeon. Nature. 1958 Mar 8;181(4610):709–711. doi: 10.1038/181709b0. [DOI] [PubMed] [Google Scholar]
- GEORGE J. C., SCARIA K. S. Histochemical demonstration of lipase activity in the pectoralis major muscle of the pigeon. Nature. 1958 Mar 15;181(4611):783–783. doi: 10.1038/181783a0. [DOI] [PubMed] [Google Scholar]
- GLOCK G. E., McLEAN P. Further studies on the properties and assay of glucose 6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase of rat liver. Biochem J. 1953 Oct;55(3):400–408. doi: 10.1042/bj0550400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAPLAN N. O. Symposium on multiple forms of enzymes and control mechanisms. I. Multiple forms of enzymes. Bacteriol Rev. 1963 Jun;27:155–169. doi: 10.1128/br.27.2.155-169.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARPATKIN S., HELMREICH E., CORI C. F. REGULATION OF GLYCOLYSIS IN MUSCLE. II. EFFECT OF STIMULATION AND EPINEPHRINE IN ISOLATED FROG SARTORIUS MUSCLE. J Biol Chem. 1964 Oct;239:3139–3145. [PubMed] [Google Scholar]
- KEECH D. B., UTTER M. F. PYRUVATE CARBOXYLASE. II. PROPERTIES. J Biol Chem. 1963 Aug;238:2609–2614. [PubMed] [Google Scholar]
- KLINGENBERG M., BUECHER T. Biological oxidations. Annu Rev Biochem. 1960;29:669–708. doi: 10.1146/annurev.bi.29.070160.003321. [DOI] [PubMed] [Google Scholar]
- KREBS H. A., WOODFORD M. FRUCTOSE 1, 6-DIPHOSPHATASE IN STRIATED MUSCLE. Biochem J. 1965 Feb;94:436–445. doi: 10.1042/bj0940436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan N. O., Goodfriend T. L. Role of the two types of lactic dehydrogenase. Adv Enzyme Regul. 1964;2:203–212. doi: 10.1016/s0065-2571(64)80014-5. [DOI] [PubMed] [Google Scholar]
- LAWRIE R. A. The activity of the cytochrome system in muscle and its relation to myoglobin. Biochem J. 1953 Sep;55(2):298–305. doi: 10.1042/bj0550298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANSOUR T. E. STUDIES ON HEART PHOSPHOFRUCTOKINASE. ACTIVE AND INACTIVE FORMS OF THE ENZYME. J Biol Chem. 1965 May;240:2165–2172. [PubMed] [Google Scholar]
- NEWSHOLME E. A., RANDLE P. J. Regulation of glucose uptake by muscle. 5. Effects of anoxia, insulin, adrenaline and prolonged starving on concentrations of hexose phosphates in isolated rat diaphragm and perfused isolated rat heart. Biochem J. 1961 Sep;80:655–662. doi: 10.1042/bj0800655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsholme E. A., Randle P. J. Regulation of glucose uptake by muscle. 6. Fructose 1,6-diphosphatase activity of rat heart and rat diaphragm. Biochem J. 1962 May;83(2):387–392. doi: 10.1042/bj0830387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsholme E. A., Randle P. J. Regulation of glucose uptake by muscle. 7. Effects of fatty acids, ketone bodies and pyruvate, and of alloxan-diabetes, starvation, hypophysectomy and adrenalectomy, on the concentrations of hexose phosphates, nucleotides and inorganic phosphate in perfused rat heart. Biochem J. 1964 Dec;93(3):641–651. doi: 10.1042/bj0930641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARMEGGIANI A., BOWMAN R. H. REGULATION OF PHOSPHOFRUCTOKINASE ACTIVITY BY CITRATE IN NORMAL AND DIABETIC MUSCLE. Biochem Biophys Res Commun. 1963 Aug 1;12:268–273. doi: 10.1016/0006-291x(63)90294-8. [DOI] [PubMed] [Google Scholar]
- PASSONNEAU J. V., LOWRY O. H. Phosphofructokinase and the Pasteur effect. Biochem Biophys Res Commun. 1962 Feb 20;7:10–15. doi: 10.1016/0006-291x(62)90134-1. [DOI] [PubMed] [Google Scholar]
- REEVES R. B. Control of glycogen utilization and glucose uptake in the anaerobic turtle heart. Am J Physiol. 1963 Jul;205:23–29. doi: 10.1152/ajplegacy.1963.205.1.23. [DOI] [PubMed] [Google Scholar]
- REGEN D. M., DAVIS W. W., MORGAN H. E., PARK C. R. THE REGULATION OF HEXOKINASE AND PHOSPHOFRUCTOKINASE ACTIVITY IN HEART MUSCLE. EFFECTS OF ALLOXAN DIABETES, GROWTH HORMONE, CORTISOL, AND ANOXIA. J Biol Chem. 1964 Jan;239:43–49. [PubMed] [Google Scholar]
- SACKTOR B. The role of mitochondria in respiratory metabolism of flight muscle. Annu Rev Entomol. 1961;6:103–130. doi: 10.1146/annurev.en.06.010161.000535. [DOI] [PubMed] [Google Scholar]
- STICKLAND R. G. Some properties of oxaloacetate-synthesizing enzyme. Biochem J. 1959 Dec;73:660–665. doi: 10.1042/bj0730660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STICKLAND R. G. Some properties of the malic enzyme of pigeon liver. 1. Conversion of malate into pyruvate. Biochem J. 1959 Dec;73:646–654. doi: 10.1042/bj0730646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNDERWOOD A. H., NEWSHOLME E. A. PROPERTIES OF PHOSPHOFRUCTOKINASE FROM RAT LIVER AND THEIR RELATION TO THE CONTROL OF GLYCOLYSIS AND GLUCONEOGENESIS. Biochem J. 1965 Jun;95:868–875. doi: 10.1042/bj0950868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNDERWOOD A. H., NEWSHOLME E. A. SOME PROPERTIES OF FRUCTOSE 1,6-DIPHOSPHATASE OF RAT LIVER AND THEIR RELATION TO THE CONTROL OF GLUCONEOGENESIS. Biochem J. 1965 Jun;95:767–774. doi: 10.1042/bj0950767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UTTER M. F. The role of CO2 fixation in carbohydrate utilization and synthesis. Ann N Y Acad Sci. 1959 Feb 6;72(12):451–461. doi: 10.1111/j.1749-6632.1959.tb44173.x. [DOI] [PubMed] [Google Scholar]
- WILSON A. C., CAHN R. D., KAPLAN N. O. Functions of the two forms of lactic dehydrogenase in the breast muscle of birds. Nature. 1963 Jan 26;197:331–334. doi: 10.1038/197331a0. [DOI] [PubMed] [Google Scholar]
- YOUNG H. L., PACE N. Distribution of alpha-glycerophosphate dehydrogenase in normal rats. Arch Biochem Biophys. 1958 Jul;76(1):112–121. doi: 10.1016/0003-9861(58)90125-5. [DOI] [PubMed] [Google Scholar]


