Abstract
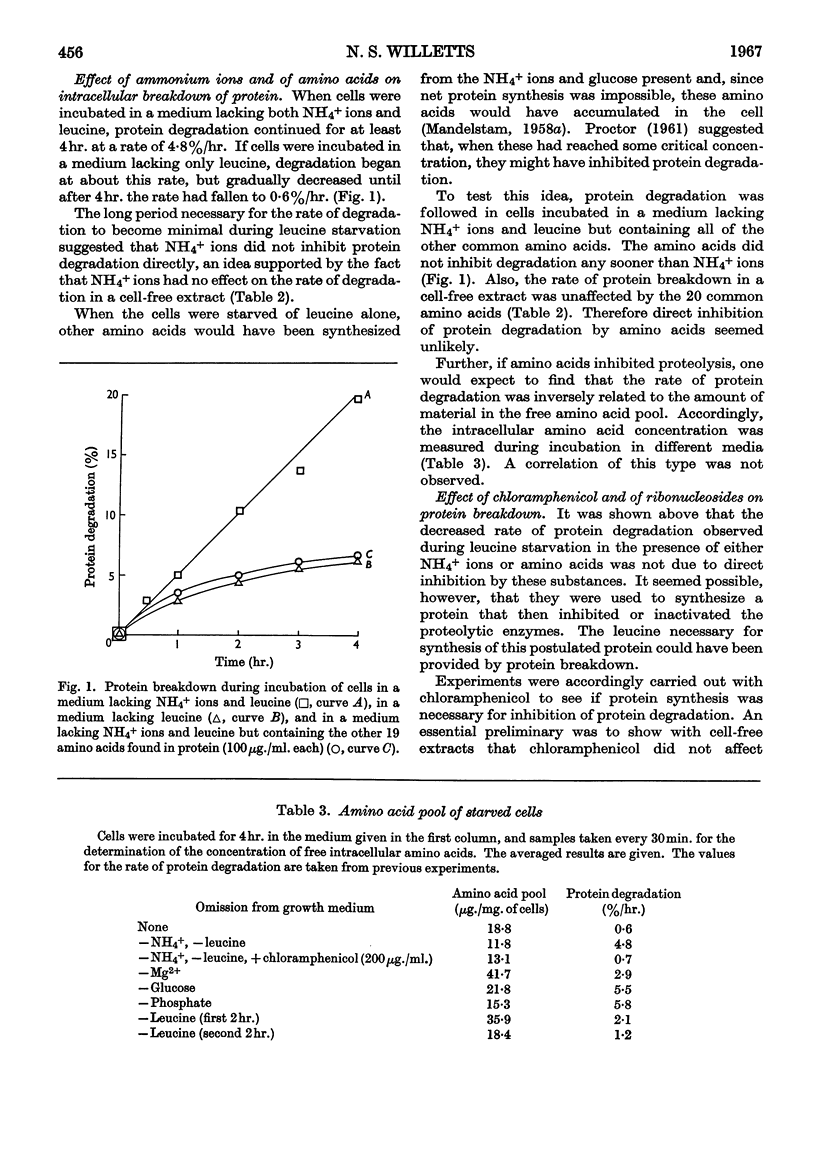
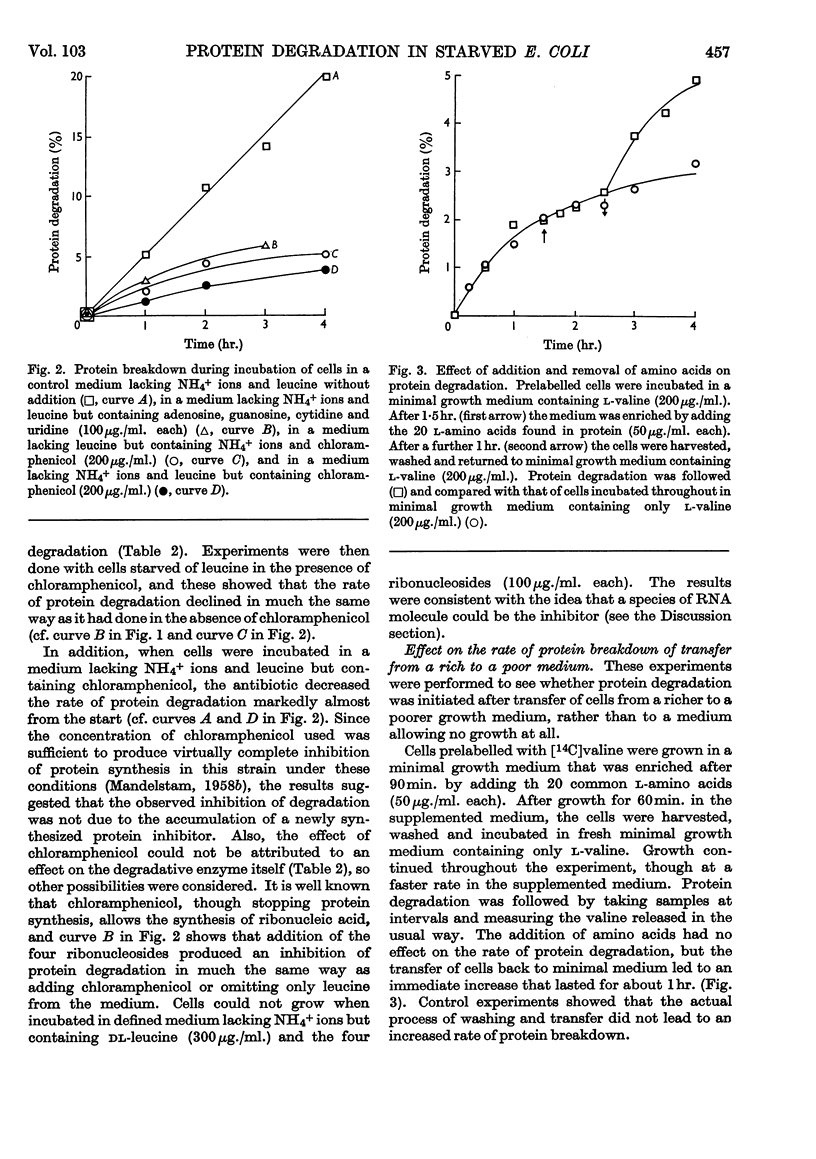
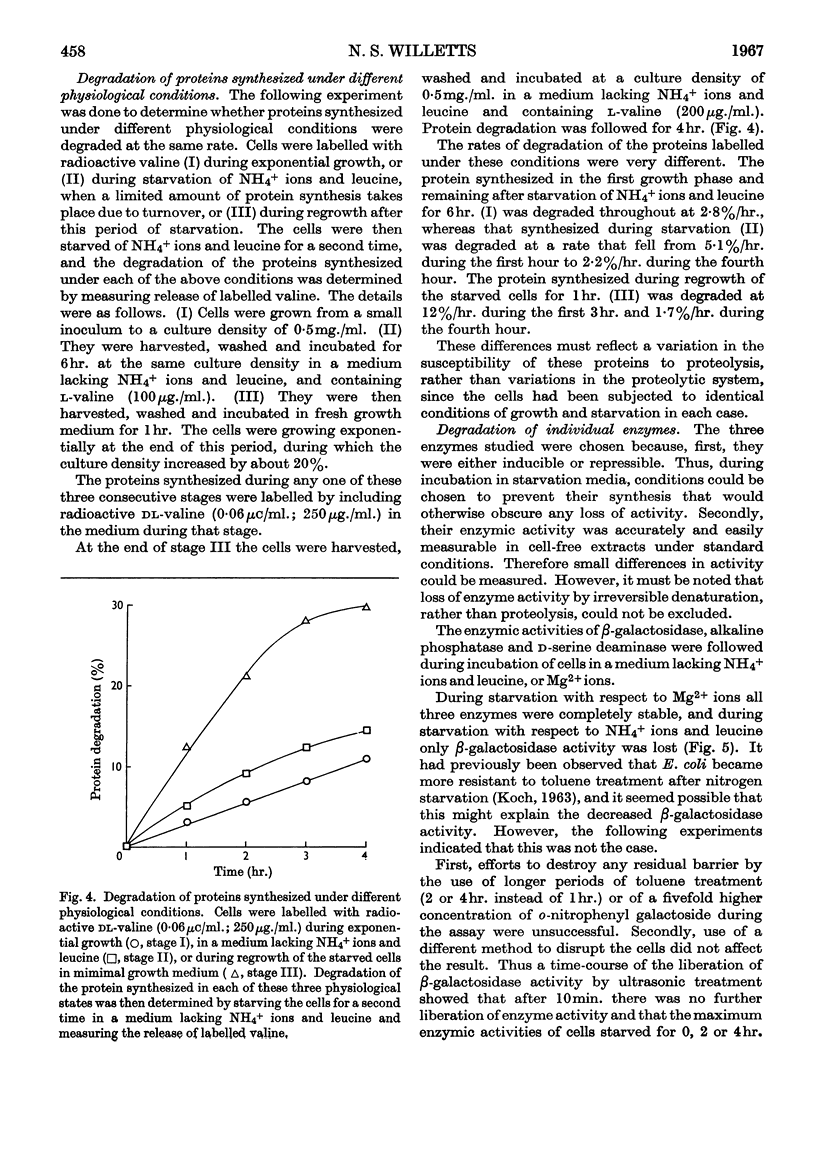
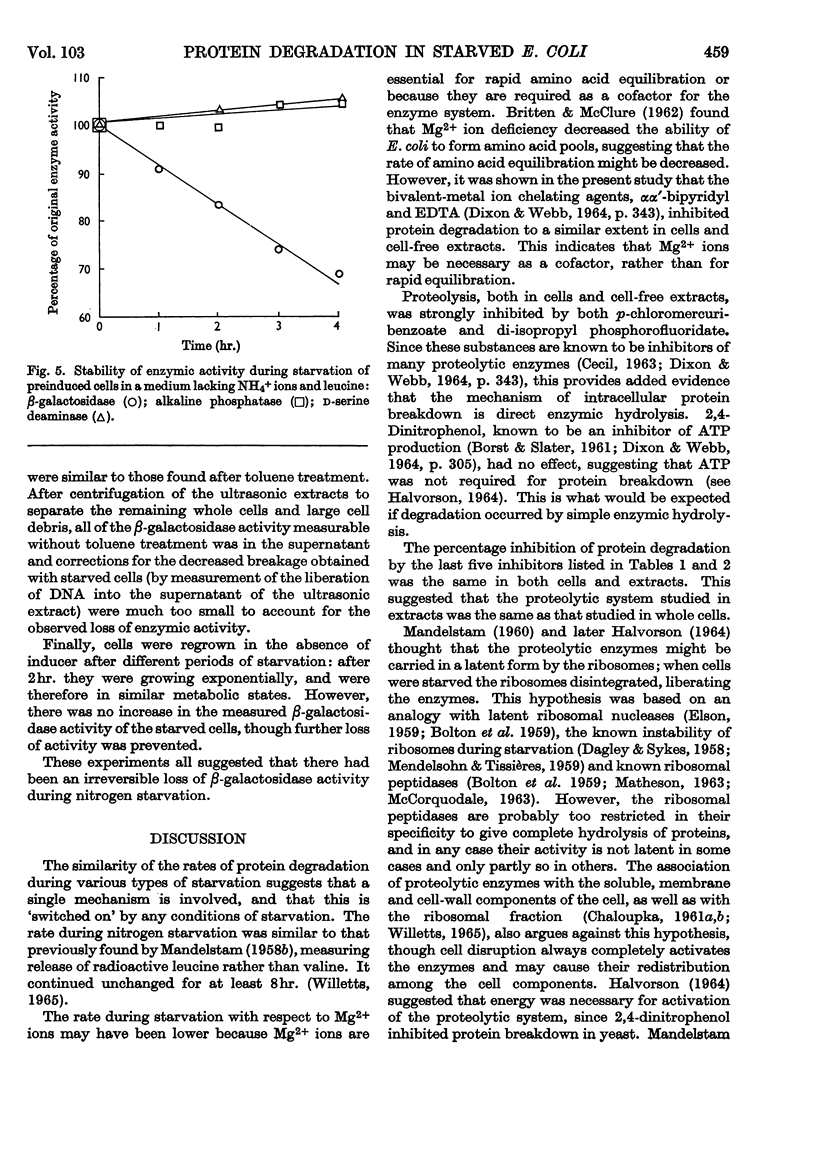
1. When Escherichia coli leu− was incubated at 35° in a medium based on minimal medium, but with the omission of phosphate ions, or glucose, or NH4+ ions and leucine, intracellular protein was degraded at a rate of about 5%/hr. in each case. If Mg2+ ions were omitted, however, the rate of degradation was 2·9%/hr. 2. Under certain conditions of incubation, protein degradation was inhibited. The inhibitor was neither NH4+ ions nor amino acids, and its properties were not those of a protein, but it might be an unstable species of RNA. 3. Although a large part of the cell protein was degraded at about 5%/hr. during starvation of NH4+ ions and leucine, some proteins were lost at more rapid rates, whereas others were lost at lower rates or not at all. 4. In particular, β-galactosidase activity was lost at about 8%/hr. during starvation of NH4+ ions and leucine, whereas d-serine-deaminase and alkaline-phosphatase activities were stable. During starvation of Mg2+ ions, all three enzyme activities were stable.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRITTEN R. J., McCLURE F. T. The amino acid pool in Escherichia coli. Bacteriol Rev. 1962 Sep;26:292–335. doi: 10.1128/br.26.3.292-335.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borek E., Ponticorvo L., Rittenberg D. PROTEIN TURNOVER IN MICRO-ORGANISMS. Proc Natl Acad Sci U S A. 1958 May;44(5):369–374. doi: 10.1073/pnas.44.5.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHALOUPKA J. Localization of a protease in the cell of Escherichia coli. Nature. 1961 Feb 11;189:512–512. doi: 10.1038/189512a0. [DOI] [PubMed] [Google Scholar]
- DAWES E. A., RIBBONS D. W. SOME ASPECTS OF THE ENDOGENOUS METABOLISM OF BACTERIA. Bacteriol Rev. 1964 Jun;28:126–149. doi: 10.1128/br.28.2.126-149.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELSON D. Latent enzymic activity of a ribonucleoprotein isolated from Escherichia coli. Biochim Biophys Acta. 1959 Dec;36:372–386. doi: 10.1016/0006-3002(59)90179-9. [DOI] [PubMed] [Google Scholar]
- Ezekiel D. H. Intracellular charging of soluble ribonucleic acid in Escherichia coli subjected to isoleucine starvation and chloramphenicol treatment. Biochem Biophys Res Commun. 1964;14:64–68. doi: 10.1016/0006-291x(63)90212-2. [DOI] [PubMed] [Google Scholar]
- KOCH A. L. The inactivation of the transport mechanism for beta-galactosides of Escherichia coli under various physiological conditions. Ann N Y Acad Sci. 1963 Jan 21;102:602–620. doi: 10.1111/j.1749-6632.1963.tb13663.x. [DOI] [PubMed] [Google Scholar]
- MANDELSTAM J. Induced biosynthesis of lysine decarboxylase in Bacterium cadaveris. J Gen Microbiol. 1954 Dec;11(3):426–437. doi: 10.1099/00221287-11-3-426. [DOI] [PubMed] [Google Scholar]
- MANDELSTAM J. The free amino acids in growing and non-growing populations of Escherichia coli. Biochem J. 1958 May;69(1):103–110. doi: 10.1042/bj0690103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANDELSTAM J. The intracellular turnover of protein and nucleic acids and its role in biochemical differentiation. Bacteriol Rev. 1960 Sep;24(3):289–308. doi: 10.1128/br.24.3.289-308.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANDELSTAM J. Turnover of protein in growing and non-growing populations of Escherichia coli. Biochem J. 1958 May;69(1):110–119. doi: 10.1042/bj0690110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANDELSTAM J. Turnover of protein in starved bacteria and its relationship to the induced synthesis of enzyme. Nature. 1957 Jun 8;179(4571):1179–1181. doi: 10.1038/1791179a0. [DOI] [PubMed] [Google Scholar]
- MATHESON A. T. The localization and properties of anaminopeptidase in Escherichia coli B. Can J Biochem Physiol. 1963 Jan;41:9–18. [PubMed] [Google Scholar]
- MCCORQUODALE D. J. SOME PROPERTIES OF A RIBOSOMAL CYSTEINYLGLYCINASE OF ESCHERICHIA COLI B. J Biol Chem. 1963 Dec;238:3914–3920. [PubMed] [Google Scholar]
- PARDEE A. B., PRESTIDGE L. S. Induced formation of serine and threonine deaminases by Escherichia coli. J Bacteriol. 1955 Dec;70(6):667–674. doi: 10.1128/jb.70.6.667-674.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POLLOCK M. R. The measurement of the liberation of penicillinase from Bacillus subtilis. J Gen Microbiol. 1961 Oct;26:239–253. doi: 10.1099/00221287-26-2-239. [DOI] [PubMed] [Google Scholar]
- PROCTOR M. H. Protein turnover in resting bacteria. Folia Microbiol (Praha) 1962 Jul;7:207–215. doi: 10.1007/BF02930765. [DOI] [PubMed] [Google Scholar]
- Pine M. J. Heterogeneity of protein turnover in Escherichia coli. Biochim Biophys Acta. 1965 Jul 8;104(2):439–456. doi: 10.1016/0304-4165(65)90349-1. [DOI] [PubMed] [Google Scholar]
- RICKENBERG H. V., LESTER G. The preferential synthesis of beta-galactosidase in Escherichia coli. J Gen Microbiol. 1955 Oct;13(2):279–284. doi: 10.1099/00221287-13-2-279. [DOI] [PubMed] [Google Scholar]
- TORRIANI A. Influence of inorganic phosphate in the formation of phosphatases by Escherichia coli. Biochim Biophys Acta. 1960 Mar 11;38:460–469. doi: 10.1016/0006-3002(60)91281-6. [DOI] [PubMed] [Google Scholar]
- Willetts N. S. Intracellular protein breakdown in growing cells of Escherichia coli. Biochem J. 1967 May;103(2):462–466. doi: 10.1042/bj1030462. [DOI] [PMC free article] [PubMed] [Google Scholar]


