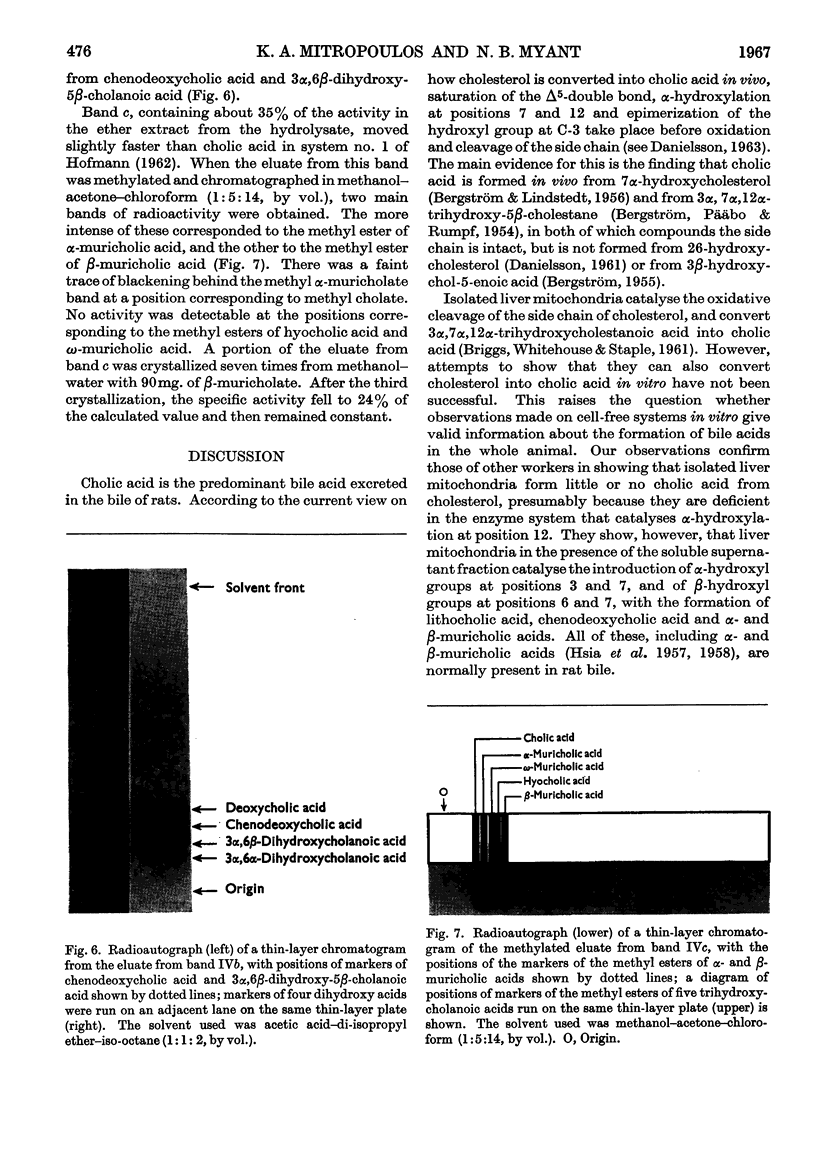
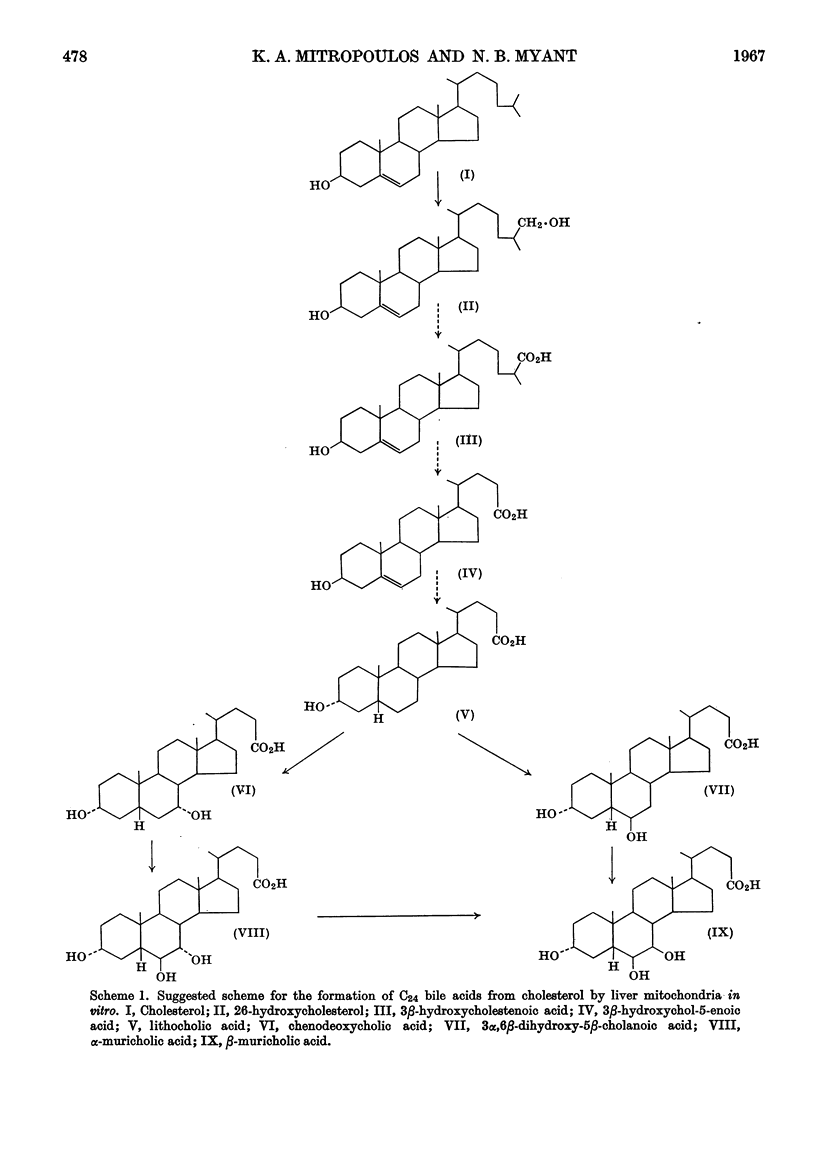
Abstract
1. When rat-liver mitochondria were incubated with [4-14C]cholesterol in the presence of a soluble supernatant fraction, various steroids more polar than cholesterol were formed. These included 3β-hydroxycholest-5-en-26-oic acid, 3β-hydroxychol-5-enoic acid, lithocholic acid, chenodeoxycholic acid and α- and β-muricholic acids. 2. All the radioactive C24 bile acids recovered were in conjugated form, probably as taurine conjugates. 3. The formation of 3β-hydroxychol-5-enoic acid from cholesterol shows that liver mitochondria are capable of carrying out the oxidative removal of the isopropyl unit of the side chain before any modification has occurred in the ring system.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVIGAN J., GOODMAN D. S., STEINBERG D. THIN-LAYER CHROMATOGRAPHY OF STEROLS AND STEROIDS. J Lipid Res. 1963 Jan;4:100–101. [PubMed] [Google Scholar]
- BERGSTROM S., LINDSTEDT S. The formation of cholic acid from 3alpha, 7alpha-dihydroxycoprostane in the rat. Biochim Biophys Acta. 1956 Mar;19(3):556–557. doi: 10.1016/0006-3002(56)90485-1. [DOI] [PubMed] [Google Scholar]
- DANIELSSON H. PRESENT STATUS OF RESEARCH ON CATABOLISM AND EXCRETION OF CHOLESTEROL. Adv Lipid Res. 1963;1:335–385. doi: 10.1016/b978-1-4831-9937-5.50015-6. [DOI] [PubMed] [Google Scholar]
- FREDRICKSON D. S., ONO K. The in vitro production of 25- and 26-hydroxycholesterol and their in vivo metabolism. Biochim Biophys Acta. 1956 Oct;22(1):183–184. doi: 10.1016/0006-3002(56)90236-0. [DOI] [PubMed] [Google Scholar]
- FREDRICKSON D. S. The conversion of cholesterol-4-C14 to acids and other products by liver mitochondria. J Biol Chem. 1956 Sep;222(1):109–120. [PubMed] [Google Scholar]
- HSIA S. L., MATSCHINER J. T., MAHOWALD T. A., ELLIOTT W. H., DOISY E. A., Jr, THAYER S. A., DOISY E. A. Bile acids. VII. Structure of acid I. J Biol Chem. 1958 Feb;230(2):573–580. [PubMed] [Google Scholar]
- HSLA S. L., MATSCHINER J. T., MAHOWALD T. A., ELLIOTT W. H., DOISY E. A., Jr, THAYER S. A., DOISY E. A. Bile acids. VI. The structure and synthesis of acid II. J Biol Chem. 1957 Jun;226(2):667–671. [PubMed] [Google Scholar]
- MOSBACH E. H., KALINSKY H. J., HALPERN E., KENDALL F. E. Determination of deoxycholic and cholic acids in bile. Arch Biochem Biophys. 1954 Aug;51(2):402–410. doi: 10.1016/0003-9861(54)90495-6. [DOI] [PubMed] [Google Scholar]
- Mitropoulos K. A., Myant N. B. The metabolism of cholesterol in the presence of liver mitochondria from normal and thyroxine-treated rats. Biochem J. 1965 Mar;94(3):594–603. doi: 10.1042/bj0940594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAY P. D., DOISY E. A., Jr, MATSCHINER J. T., HSIA S. L., ELLIOTT W. H., THAYER S. A., DOISY E. A. Bile acids. XVI. Metabolism of cholanic acid-24-C-14 in the rat. J Biol Chem. 1961 Dec;236:3158–3162. [PubMed] [Google Scholar]
- SIPERSTEIN M. D., HAROLD F. M., CHAIKOFF I. L., DAUBEN W. G. C14-Cholesterol. VI. Biliary end-products of cholesterol metabolism. J Biol Chem. 1954 Sep;210(1):181–191. [PubMed] [Google Scholar]
- SIPERSTEIN M. D., MURRAY A. W. Enzymatic synthesis of cholyl coA and taurocholic acid. Science. 1956 Mar 2;123(3192):377–378. doi: 10.1126/science.123.3192.377. [DOI] [PubMed] [Google Scholar]
- THOMAS P. J., HSIA S. L., MATSCHINER J. T., DOISY E. A., Jr, ELLIOTT W. H., THAYER S. A., DOISY E. A. BILE ACIDS. XIX. METABOLISM OF LITHOCHOLIC ACID-24-14C IN THE RAT. J Biol Chem. 1964 Jan;239:102–105. [PubMed] [Google Scholar]
- THOMAS P. J., HSIA S. L., MATSCHINER J. T., THAYER S. A., ELLIOTT W. H., DOISY E. A., Jr, DOISY E. A. BILE ACIDS. XXI. METABOLISM OF 3-ALPHA,6-BETA-DIHYDROXY-5-BETA-CHOLANOIC ACID-24-C 14-6-ALPHA-H3 IN THE RAT. J Biol Chem. 1965 Mar;240:1059–1063. [PubMed] [Google Scholar]
- WAGNER H., HOERHAMMER L., WOLFF P. [Thin layer chromatography of phosphatides and glycolipids]. Biochem Z. 1961;334:175–184. [PubMed] [Google Scholar]
- WHITEHOUSE M. W., STAPLE E., GURIN S. Catabolism in vitro of cholesterol. I. Oxidation of the terminal methyl groups of cholesterol to carbon dioxide by rat liver preparations. J Biol Chem. 1959 Feb;234(2):276–281. [PubMed] [Google Scholar]