Abstract
The elimination of viral covalently closed circular DNA (CCC DNA) from the nucleus of infected hepatocytes is an obstacle to achieving sustained viral clearance during antiviral therapy of chronic hepatitis B virus (HBV) infection. The aim of our study was to determine whether treatment with adefovir, a new acyclic nucleoside phosphonate, the prodrug of which, adefovir dipivoxil, is in clinical evaluation, is able to suppress viral CCC DNA both in vitro and in vivo using the duck HBV (DHBV) model. First, the effect of adefovir on viral CCC DNA synthesis was examined with primary cultures of DHBV-infected fetal hepatocytes. Adefovir was administered for six consecutive days starting one day before or four days after DHBV inoculation. Dose-dependent inhibition of both virion release in culture supernatants and synthesis of intracellular viral DNA was observed. Although CCC DNA amplification was inhibited by adefovir, CCC DNA was not eliminated by antiviral treatment and the de novo formation of CCC DNA was not prevented by pretreatment of the cells. Next, preventive treatment of experimentally infected ducklings with lamivudine or adefovir revealed that both efficiently suppressed viremia and intrahepatic DNA. However, persistence of viral DNA even when detectable only by PCR was associated with a recurrence of viral replication following drug withdrawal. Taken together, our results demonstrate that adefovir is a potent inhibitor of DHBV replication that inhibits CCC DNA amplification but does not effectively prevent the formation of CCC DNA from incoming viral genomes.
Despite the existence of efficient vaccines, chronic hepatitis B virus (HBV) infection continues to be a major public health problem worldwide, with more than 350 million chronic carriers. These individuals are at high risk of developing cirrhosis and hepatocellular carcinoma (28). Interferon alpha therapy is only moderately effective and often is limited by dose-dependent side effects (20). The discovery that certain nucleoside inhibitors of human immunodeficiency virus reverse transcriptase, such as lamivudine, also inhibit HBV polymerase has led to the development of these agents for the treatment of HBV infection. Lamivudine has been shown to be highly effective in inhibiting HBV replication (10, 25) and has recently been licensed in many countries for the therapy of chronic hepatitis B. However, analysis of the kinetics of viral clearance during lamivudine therapy revealed that since lamivudine does not completely inhibit viral replication and the rate of clearance of infected cells is slow, prolonged therapy is required for elimination of virus (38). The initial reactions required for the conversion of the incoming relaxed circular (RC) DNA into covalently closed circular (CCC DNA) are still not elucidated, but it can be hypothesized that HBV polymerase (23) and cellular enzymes (2) may be required for this process. CCC DNA serves as the template for viral transcription (46), and its production is regulated and amplified by an intracellular pathway in which newly synthesized genomic DNA is recycled to the nucleus (47). This process establishes a pool of nuclear CCC DNA, which is maintained during the life of infected cells. HBV is not a cytopathogenic virus, and the viral CCC DNA persists in the nuclei of infected cells, as long as the hepatocytes survive (34), explaining the requirement for long-term antiviral treatment. Since prolonged lamivudine therapy is associated with the selection of drug-resistant mutants (50), new nucleoside analogues should be evaluated with special emphasis on their effects on the formation of CCC DNA and the clearance kinetics of the recalcitrant viral CCC DNA during the therapy.
Adefovir [9-(2-phosphonylmethoxyethyl) adenine] is an acyclic phosphonate nucleotide analog of deoxyadenosine monophosphate which, unlike nucleoside analogs, does not require the first of three phosphorylation steps for conversion to the active triphosphate form. Adefovir diphosphate, the active metabolite of adefovir, inhibits both human immunodeficiency virus reverse transcriptase and HBV polymerase and has been shown to inhibit the lamivudine-resistant mutants as well as wild-type HBV polymerase (42, 48). In vitro, adefovir was a potent inhibitor of viral replication in human hepatoma cell lines stably transfected with HBV and in primary duck hepatocytes infected with duck HBV (DHBV) (19). The antihepadnaviral activity of adefovir was also demonstrated in vivo in the duck model with a rapid sustained antiviral response during treatment, followed by a relapse of viral replication following drug withdrawal (18). Moreover, in phase II clinical trials, adefovir dipivoxil, the oral prodrug of adefovir (PMEA), caused a greater than 4.1 log10 reduction in HBV DNA after 12 weeks of treatment in patients with chronic HBV infection (44). Analysis of the clearance kinetics of HBV during therapy implied that viral replication was inhibited more efficiently in patients treated with adefovir dipivoxil (44) than in those treated with lamivudine (38). One hypothesis is that adefovir may exhibit an inhibitory effect on the initial steps of viral infection, including CCC DNA formation, in uninfected hepatocytes to explain these differences in viral clearance kinetics.
In this study, we analyzed the kinetics of formation and elimination of CCC DNA during adefovir therapy and examined the capacity of adefovir to protect naive hepatocytes from infection by residual circulating virions in comparison to lamivudine by using the duck model of HBV infection. Our results show that adefovir is a more potent inhibitor of DHBV replication than lamivudine in experimentally infected ducklings in vivo and primary duck hepatocytes in vitro, but it does not prevent the initiation of infection.
MATERIALS AND METHODS
Drugs.
Adefovir (PMEA) was supplied by Gilead Sciences, Foster City, Calif. Stock solutions of lamivudine (Glaxo SmithKline) and adefovir were prepared in phosphate-buffered saline, and the dilutions of drugs were added to cell culture medium immediately before each medium change. Two drug concentrations, 5 and 15 mg/ml, obtained by dissolving adefovir in phosphate-buffered saline and adjusted to pH to 7.3, were used for in vivo study.
Primary duck hepatocyte cultures.
Hepatocytes were isolated from uninfected duck embryos (2). The embryos were sacrificed by decapitation a week before the hatch. The liver was prepared by digestion with 0.2% collagenase (type V, 230 units/mg; Sigma), and DNase I and hepatocytes were purified by the Percoll method. Hepatocytes were seeded on six-well plates at a density of 5 × 105 cells per well in hepatocyte attachment medium. After 5 h, the medium was replaced by William medium E with dimethyl sulfoxide (1.5%) (Sigma), kanamycin (50 U/ml) (Gibco BRL), penicillin-streptomycin (50 U/ml) (Gibco BRL), bovine insulin (5 μg/ml) (Roche Diagnostics, Mannheim, Germany), and dexamethasone (0.04 μg/ml) (Soludecadron; Merck Sharp and Dohme-Chibret Laboratories). The cell cultures were maintained in a 5% CO2 atmosphere at 37°C. The culture medium was changed daily. Hepatocytes were infected with a DHBV-positive serum (2 × 109 to 9 × 109 genome equivalents per well) as previously described (3, 45).
In vitro treatment and cytotoxicity.
To determine the effect of adefovir administration on viral DNA synthesis, antiviral treatment was started 4 days after infection, using 3 concentrations (0.1, 1, and 10 μM) of adefovir or lamivudine, and was maintained for 7 days. To examine the capacity of drugs to inhibit the initial steps of viral infection including CCC DNA formation and its amplification, the drugs were added to hepatocyte culture medium, at the same concentrations as previously, 1 day before DHBV infection and on the day of inoculation and were maintained for 5 or 9 days postinfection.
Cellular toxicity was tested by daily light microscope examination and by incorporation of neutral red. Briefly, hepatocytes of duck embryos were seeded on 24-well plates and cultured in medium containing increasing concentrations of adefovir for 7 days, with a daily change of medium. Cell viability was estimated according to a protocol already described (12). After incubation in medium containing 1/80 neutral red dye for 3 h at 37°C, the cells were fixed in a 4% formaldehyde-1% calcium chloride solution and finally lysed in an acetic acid ethanol mixture. The fifty percent cytoxic concentration was defined as the drug concentration required to reduce cell viability by 50% in triplicate assays.
Experimental infection of ducklings.
Ducklings were maintained under normal daylight and fed with a standard commercial diet and water ad libitum, in accordance with the guidelines for animal care at the facilities of the National Veterinary School of Lyon, Marcy l'Etoile, France. Five-day-old ducklings were inoculated intravenously with a DHBV-positive serum specimen known to be infectious, and each duckling received 107 viral genome equivalents by following a protocol previously described (1, 29). Ducklings received adefovir by intraperitoneal administration and received lamivudine orally. Adefovir was used intraperitoneally because it has the same effect as the prodrug, adefovir dipivoxil, which is transformed in adefovir, found in the circulation as demonstrated in primate studies (8). Adefovir was used intraperitoneally at a dose of 15 mg/kg of body weight/day in our studies as previously described by Nicoll et al. (37). The treatment began 1 day before the infection and was maintained for 3 weeks according to the protocols described in Results. Viremia, animal weight, and lactic acid levels were monitored throughout the study period.
Analysis of viral DNA.
DHBV DNA from the serum of experimentally infected ducklings and from hepatocyte culture supernatants was detected by a specific dot blot hybridization assay, 50 μl of serum or 600 μl of culture supernatants were spotted, and DHBV DNA was detected with a full-length DHBV genomic DNA probe labeled with [α-32P]dCTP as described previously (26). A quantitative analysis was carried out using a PhosphorImager SI system. The limit of detection of serum viral DNA by this assay is 100 pg/ml.
Total DNA and CCC DNA from primary hepatocytes were isolated as described by Summers et al. (43). CCC DNA preparations as well as an equivalent volume of the corresponding replicative intermediate DNA preparations were analyzed by electrophoresis through a 1.2% agarose gel, followed by Southern blot analysis.
Intrahepatic viral DNA from experimentally infected duckling was extracted by a procedure described by Jilbert et al. (21). Liver samples were snap-frozen in liquid nitrogen and were stored at −80°C and then analyzed for viral DNA. One hundred milligrams of liver was homogenized in 0.01 M Tris-HCl (pH 7.5)-0.01 M EDTA. The homogenate was divided into two parts, one for the isolation of total viral DNA and one for the isolation of non-protein-bound, viral CCC DNA. DNA preparations were analyzed by Southern blot as previously described (29).
The membranes were analyzed by autoradiography and were read with the PhosphorImager System SI. Quantitative image analysis was performed with ImageQuant software. The Mann-Whitney test was used to compare the different groups of animals. Differences were considered statistically significant when the P value was <0.05.
In serum samples with undetectable DHBV DNA by the dot blot assay, viral DNA was extracted with the High Pure PCR template Preparation Kit (Roche). PCR detection of total viral DNA was performed with a specific primer pair: Primer P1 (5"-GCG CTT TCC AAG ATA CTG GAG CCC AA-3") at nucleoside positions 1426 to 1451 and primer P2 (5"-CTG GAT GGG CCG TCA GCA GGA TTA TA-3") at nucleotide positions 2445 to 2420. Intrahepatic viral DNA was also amplified by PCR in selected samples using primer pair P1 and P2 as well as primer pair P1 and P3 (5"-CCC TGT GTA GTC TGC CAG AAG TCT TC-3", nucleotide positions 2843 to 2818) to amplify the gap region of the viral genome, which is completely double-stranded only in CCC DNA. After 30 amplification cycles (1 min, 94°C; 3 min, 72°C), PCR products were separated through 1.5% agarose gels.
RESULTS
Adefovir inhibits viral DNA synthesis in primary duck hepatocyte cultures.
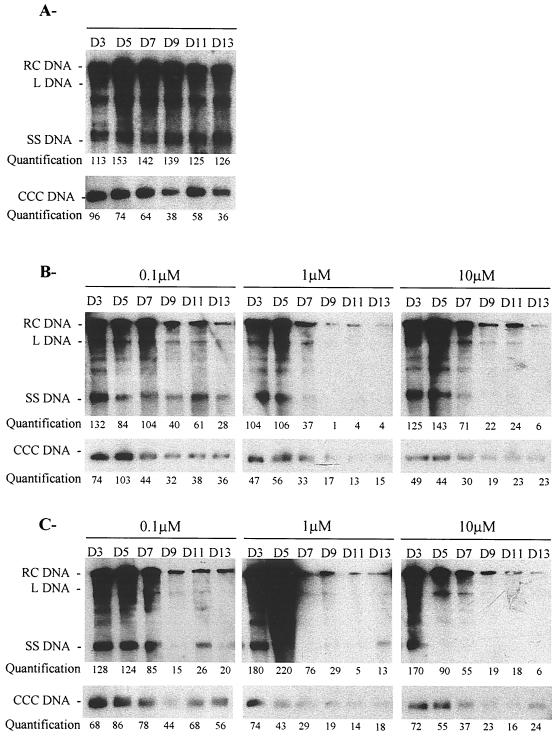
We first examined whether adefovir inhibits viral DNA synthesis and reduces the pool of nuclear viral CCC DNA. Adefovir and lamivudine were administered, at the same concentrations, to primary duck hepatocytes experimentally infected with DHBV. The drugs were added at increasing concentrations, from 0.1 to 10 μM, to the culture medium 4 days after DHBV infection and were maintained for 7 days with a daily medium change. The medium was collected daily for quantitation of virion DNA by dot blot analysis, and intracellular viral DNA was analyzed by a Southern blot every 2 days after infection. Quantification of DHBV DNA in the culture medium indicated that virion secretion was reproducibly inhibited by adefovir and lamivudine in a concentration-dependent manner. After seven days of drug treatment, the 50% inhibitory concentration IC50 of adefovir (0.01 μM) was lower than that of lamivudine (0.1 μM) (data not shown). Southern blot analysis of the viral replicative intermediates and CCC DNA was performed from day 3 to day 13 after infection (Fig. 1).The magnitude and kinetics of the inhibition of viral replicative intermediates and CCC DNA were concentration dependent. However, viral CCC DNA was not eliminated from infected hepatocytes during adefovir and lamivudine treatment. No relapse of viral replication was observed at day 13 postinoculation, 2 days after drug withdrawal.
FIG. 1.
Adefovir inhibits viral DNA synthesis in primary duck hepatocyte cultures. Primary duck hepatocyte cultures were inoculated with an infectious serum on the day of seeding (day 0). Intracellular viral DNA was analyzed by Southern blotting and specific hybridization at the indicated time points during cell culture. Adefovir or lamivudine was added from day 4 postinoculation, at the indicated concentrations, for seven consecutive days with a daily medium change. The viral replicative intermediates are indicated. Days are indicated above the lanes. (A) Untreated control culture. (B) Adefovir-treated culture. (C) Lamivudine-treated culture. Relative band intensities for total DHBV DNA and for CCC DHBV DNA were quantified by PhosphoImager scanning; the results are presented in arbitrary units. L DNA, linear DNA; SS DNA, single-stranded DNA.
Adefovir does not prevent initiation of infection in primary duck hepatocyte cultures.
Experiments were then carried out to examine whether adefovir has the capacity to block the initiation of infection, defined as the conversion of virion DNA into CCC DNA. Adefovir and lamivudine were added to the culture medium 1 day before DHBV infection, and the medium was maintained for 5 or 9 days postinoculation. The virion DNA in the medium was analyzed daily by a dot blot, and intracellular viral DNA was analyzed every other day by a Southern blot. In the first experiment, the preventive treatment was administered from day −1 (preinoculation) to day 5 postinoculation (data not shown). Dot blot analysis showed that adefovir pretreatment delayed the secretion of virions in the culture supernatants. Southern blot analysis of intracellular viral DNA revealed a dose-dependent decrease of replicative intermediates, including CCC DNA, during antiviral treatment. After cessation of treatment with adefovir and lamivudine, all viral DNA replicative forms became detectable at all concentrations. CCC DNA was detected at day 2 postinfection, the earliest time point examined, in the control and in all the treated cultures, suggesting that adefovir, like lamivudine, was not able to prevent the initial formation of CCC DNA (data not shown).
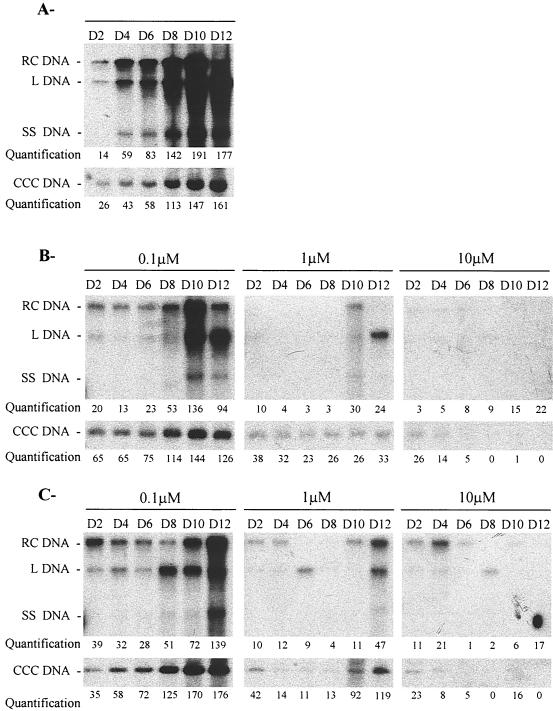
These results were confirmed when the preventive treatment was maintained for 9 days postinoculation (Fig. 2).Quantification of the virion DNA in the culture supernatant revealed a significant, concentration-dependent reduction by adefovir. The IC50 of adefovir on virion release (IC50, 0.01 μM) was 10-fold lower than that of lamivudine (IC50, 0.1 μM). CCC DNA and other viral replicative intermediates were detectable in all treated cultures at day 2 postinoculation. However, the amplification of CCC DNA was delayed and stabilized at lower levels than in control cells during therapy with low concentrations of adefovir or lamivudine but decreased over time when adefovir or lamivudine were administered at concentrations of 10μM. After treatment was stopped, the level of replicative intermediates including CCC DNA increased in primary hepatocytes treated with low concentrations of adefovir or lamivudine (0.1 and 1 μM), while no relapse was observed with higher concentrations (10 μM) of adefovir or lamivudine (Fig. 2). Nevertheless, viral DNA was still detectable by PCR at the end of the treatment in adefovir and lamivudine-treated cultures, indicating the absence of clearance of virus from infected hepatocytes (data not shown). Therefore, these results suggest that under these conditions adefovir could delay and inhibit but not eliminate CCC DNA amplification during the early steps of viral infection.
FIG. 2.
Preinoculation treatment with adefovir does not prevent the initial viral CCC DNA formation in primary duck hepatocyte cultures. Two days after seeding, primary duck hepatocyte cultures were inoculated (day 0) with an infectious DHBV serum. Intracellular viral DNA was analyzed by Southern blotting and specific hybridization at the indicated time points during cell culture. Adefovir or lamivudine was added 1 day before inoculation (day −1), at the indicated concentrations, and for 11 consecutive days with a daily medium change. The viral replicative intermediates are indicated. Days are indicated above the lanes. (A) Untreated control culture. (B) Adefovir-treated culture. (C) Lamivudine-treated culture. Relative band intensities for total DHBV DNA and for CCC DHBV DNA were quantified by PhosphoImager scanning; the results are presented in arbitrary units. L DNA, linear DNA; SS DNA, single-stranded DNA.
We also wondered whether the reduction of CCC DNA levels by adefovir might be due to the loss of infected cells rather than inhibition of CCC DNA amplification. However, no significant signs of cytotoxicity were detected in hepatocytes cultured with different concentrations of adefovir for 7 or 12 days by daily microscope examination and by neutral red incorporation. The 50% cytotoxic concentration of adefovir was 200 μM after 7 days of treatment compared to 1,000 μM for lamivudine.
Preventive treatment with adefovir in vivo.
We then investigated whether prolonged administration of adefovir beginning before inoculation with infectious serum would succeed in preventing or controlling viral infection of duck.
Ten ducks received adefovir by intraperitoneal injection, 12 received oral lamivudine, and 12 were used as controls. An induction-maintenance regimen was used, and on the day before inoculation and on the day of inoculation the ducks received 30 mg of adefovir/kg/day or 100 mg of lamivudine/kg/day. The ducks then received 15 and 50 mg of adefovir and lamivudine/kg/day, respectively, for 20 days. Half of the ducks from each group were sacrificed at the end of the treatment for intrahepatic virus analysis. The remaining ducks were sacrificed and analyzed 3 weeks after treatment was stopped.
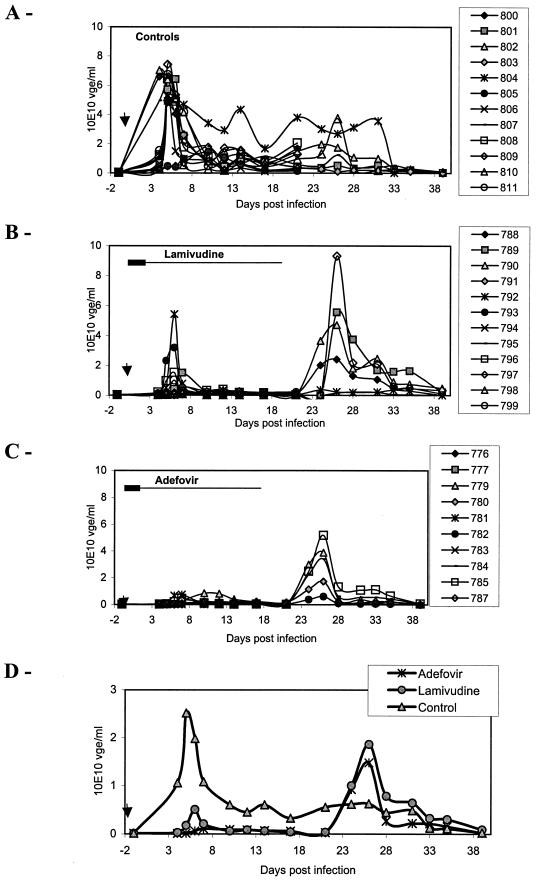
Viremia was monitored by serum dot blot hybridization throughout the study (Fig. 3).In untreated controls a peak in viremia was observed at day 5 postinfection. The peak of viremia was inhibited 80% (P = 0.0005) by lamivudine and 96% (P = 0.0002) by adefovir relative to the untreated ducks. This indicates that residual viral replication during treatment, potentially a source of resistant mutants, was 4.4-fold higher in lamivudine-treated ducks than in those treated with adefovir. In addition, the peak of viremia was delayed by 2 days and 1 day in adefovir- and lamivudine-treated ducklings, respectively, compared with the control ducklings. At the end of therapy, all the treated animals had a low level of viremia, representing an inhibition of 94% (P < 0.0001 for both drugs), compared to the levels observed for the control ducklings. In 3 out of 10 adefovir-treated ducklings and 4 out of 12 lamivudine-treated ducklings, suppression of viremia to levels undetectable by dot blot hybridization was observed at the end of the treatment. However, apart from one adefovir-treated animal, RC DNA remained detectable in the serum of these ducks using a PCR assay. Despite the profound antiviral activity of adefovir, a transient peak of viremia occurred 5 days after drug withdrawal in each adefovir-treated duckling. The peak of viremia at day 25 was not significantly different between the lamivudine-treated group and the adefovir treated group. The peak of viremia of the control group, which occurred at day 5 postinfection, was not significantly different from the peak of the lamivudine-treated group and adefovir-treated group, which occurred at day 25 postinoculation (5 days after drug withdrawal).
FIG. 3.
Preventive adefovir treatment delays the onset of viremia. The individual viremias of three groups of animals, untreated control ducks (A), lamivudine-treated ducks (B), and adefovir-treated ducks (C), as well as mean viremia of these groups (D), are represented. The animals were treated 1 day before and on the day of infection with 30 mg of adefovir/kg/day or 100 mg of lamivudine/kg/day, followed by a maintenance therapy of 15 mg of adefovir/kg/day or 50 mg of lamivudine/kg/day for 20 days. Half of the ducks were sacrificed at the end of treatment. Viremia was quantitatively analyzed by dot blot hybridization. Results of virus genome equivalent per milliliter of serum (vge/ml) in individual animals are plotted on the graph. The bar indicates the antiviral treatment period (the large bar indicates the 30-mg/kg/day adefovir or 100-mg/kg/day lamivudine regimen, and the small bar indicates the 15-mg/kg/day adefovir or 50-mg/kg/day lamivudine regimen). The arrow indicates the time of virus inoculation.
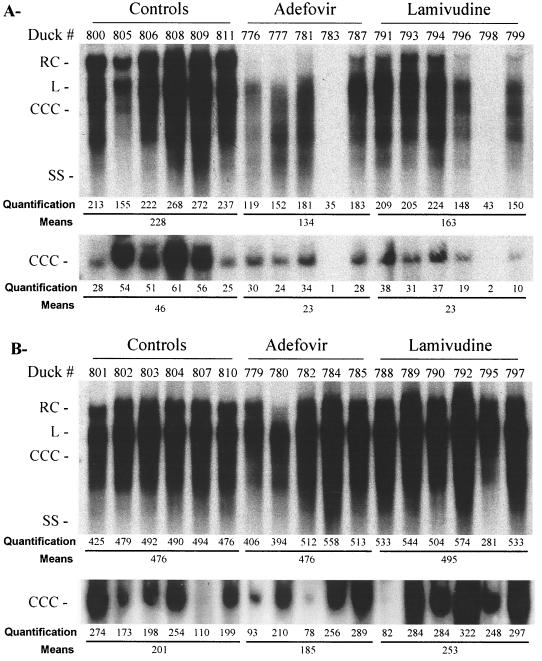
Analysis of intrahepatic viral DNA replicative intermediates by gel electrophoresis and Southern blot hybridization at the end of therapy revealed that adefovir was able to reduce intrahepatic viral DNA levels by 41% (n = 5; P = 0.0176), while the inhibition of viral DNA synthesis in lamivudine-treated animals was 28% (n = 6; P = 0.0374) compared to control animals (Fig. 4).The persistence of all viral DNA replicative intermediates as well as CCC DNA in five out of six lamivudine-treated ducklings and four out of five adefovir-treated animals indicated that antiviral therapy was not able to prevent the spread of virus within the liver, despite the significant suppression of viremia. Interestingly, viral DNA replicative intermediates as well as CCC DNA were reduced to barely detectable levels after 3 weeks of treatment in one lamivudine-treated animal and one adefovir-treated animal (Fig. 4, ducks 798 and 783, respectively). However, intrahepatic viral DNA was still detected by PCR in both animals (data not shown). Eventually, viral DNA was detected in all the treated ducks, indicating the absence of clearance of virus from the liver. These results revealed that despite the more potent antiviral effect of adefovir, viral DNA replication had been reinitiated in both adefovir- and lamivudine-treated groups.
FIG. 4.
Adefovir therapy decreases viral DNA synthesis in the liver but is not sufficient to eradicate viral infection. Total viral DNAs were extracted, including the replicative intermediates as well as viral CCC DNA (CCC). Three groups of animals were used: untreated control ducks, adefovir-treated ducks, and lamivudine-treated ducks. The animals were treated 1 day before and on the day of infection with 30 mg of adefovir/kg/day or 100 mg of lamivudine/kg/day, followed by a maintenance therapy of 15 mg of adefovir/kg/day or 50 mg/kg/day for 20 days. Half of the ducks were sacrificed at the end of treatment (A), and the other half were sacrificed at 3 weeks after the end of treatment (B). Relative band intensities for total DHBV DNA and for CCC DHBV DNA were quantified by PhosphoImager scanning for each animal and for each duck group; the results are presented in arbitrary units. RC, RC DNA; L, linear DNA; SS, single-stranded DNA.
Southern blot analysis of intrahepatic viral DNA 3 weeks after drug withdrawal showed that after this followup period, the intrahepatic levels of DHBV replicative intermediates, including CCC DNA, in treated ducks were the same as in untreated ducks.
All the ducks remained healthy regardless of treatment group. Potential side effects were monitored throughout and after cessation of therapy including animal weight and lactic acid levels. All the ducks in the study demonstrated a steady increase in weight, and the mean increases were comparable between treated and untreated groups. Determination of lactic acid levels in the plasma of the animals which received adefovir or lamivudine therapy for 3 weeks revealed no significant increases compared with the control animals.
DISCUSSION
Because CCC DNA is stably maintained in infected hepatocytes, clearance of HBV infection is dependent on the relatively slow turnover of infected cells, and antiviral therapy of chronic hepadnavirus infections with nucleoside analogs requires long-term administration to eradicate viral infection (30, 32). However, prolonged antiviral treatment with lamivudine may be associated with the selection of resistant strains in a significant proportion of patients (11, 35). Adefovir is a promising nucleotide analog that exhibits activity against wild-type HBV as well as lamivudine-resistant HBV mutants (15, 40, 42, 48). Since the kinetics of viral clearance in adefovir-treated patients were reported to be faster than with lamivudine, we investigated the effect of adefovir on CCC DNA formation and amplification in the duck model of HBV infection.
In primary hepatocyte cultures infected with DHBV in vitro, adefovir induced a concentration-dependent inhibition of viral DNA synthesis. These data are consistent with the observation of Heijtink et al. (18) and Kruining et al. (24), who also showed a potent inhibitory effect of adefovir on both DHBV and HBV replication in vitro. However, despite strong inhibition of the viral DNA replicative intermediates and viral CCC DNA synthesis, CCC DNA was not cleared from cultured hepatocytes. Our results demonstrated a greater suppression of DHBV replication by adefovir than by lamivudine, in agreement with previous data obtained with avian cells transfected with the DHBV genome (42). On the other hand, lamivudine was shown to be as effective as or more effective than adefovir in human hepatoma cell lines transfected with the HBV genome (14, 24, 39). These differences in activity may reflect differences between the species of hepadnavirus and of the cell type. In our in vivo study, adefovir was used intraperitoneally because it has a poor oral bioavailability. The intraperitoneal route makes it possible to obtain in blood circulation the same concentration of adefovir as that resulting from oral administration of the prodrug, adefovir dipivoxil (8). This in vivo study also showed that adefovir inhibits DHBV replication but is not able to eradicate viral CCC DNA from the liver, in keeping with other observations with the duck model with adefovir (18, 37) or other compounds (31, 33). Our data are also consistent with those of others, who showed, using woodchuck hepatocyte cultures treated long term with different nucleoside analogs, including lamivudine (34) and adefovir (9), that viral CCC DNA is a very stable molecule whose half-life may match that of the infected hepatocytes.
Besides the observation that adefovir administration inhibits DHBV viral DNA synthesis in hepatocytes in vitro and in vivo, our study revealed new information regarding its antiviral activity. In primary hepatocyte cultures, administration of adefovir 1 day prior to inoculation with virus delayed and inhibited DHBV genome replication and amplification of viral CCC DNA more potently than lamivudine. However, as observed with lamivudine, adefovir was unable to completely prevent the initial formation of CCC DNA. The in vivo administration of adefovir 1 day prior to viral inoculation and maintained administration of the drug for 3 weeks postinoculation confirmed our in vitro data. The results showed a more significant reduction of DHBV replication by adefovir than by lamivudine, but CCC DNA that was formed despite antiviral therapy was the source of renewed viral production after the cessation of therapy. These data suggest that in our experimental conditions, adefovir could inhibit and delay but not prevent the infection of naive hepatocytes and spread of virus in the liver. Potentially, pretreatment for more than 1 day prior to inoculation may allow time for higher concentrations of the drug and the active metabolite, adefovir diphosphate, to accumulate inside cells and confer a more potent effect. Adefovir, which inhibits both HBV and DHBV reverse transcriptase (42, 48), was unable to prevent the conversion of RC-DNA to transcriptionally active CCC DNA, suggesting that the polymerase activity of the viral reverse transcriptase may not be the only critical determinant involved in the repair reactions. Köck and Schlicht strongly suggested that cellular enzymes may be sufficient for conversion of virion RC-DNA into CCC-DNA (23). Nonetheless, their analysis was limited, like ours, to possible enzymatic targets which are blocked by nucleoside analogs. Since the viral polymerase mediates, among other functions, the transport of the hepadnavirus genome into the nucleus (22), it is possible that both cellular and viral enzymes may be involved in viral CCC DNA formation but that adefovir has no influence on the repair reaction.
Several studies have showed that viral CCC DNA clearance may involve lysis of infected cells or may be hastened by increased cell turnover (13, 17). In our primary hepatocyte culture experiments, adefovir did not exhibit significant cellular toxicity. The inhibitory effect on viral CCC DNA amplification may therefore be explained by the potent suppression of the synthesis of viral replication intermediates. Newbold et al. demonstrated the existence of two subpopulations of CCC DNA, depending on the interaction of this viral DNA form with nucleoproteins (36). They also hypothesized that these two different populations of CCC DNA may have different half-lives (5). In our in vitro and in vivo experiments, we can speculate that the unstable form of CCC DNA may explain the observation of a decline in total viral CCC DNA over time in primary embryonic duck hepatocyte culture, while the more stable form may explain the lack of complete clearance of CCC DNA both in tissue culture and in vivo.
The faster kinetics of viral clearance observed in chronic hepatitis B patients receiving adefovir therapy (44) than with lamivudine treatment (38) may therefore be better explained by a more potent inhibition of hepadnavirus genome replication and its subsequent consequences on CCC DNA amplification rather than a direct effect on the initial formation of viral CCC DNA.
In our study, although a potent reduction of viremia was observed during adefovir therapy, the persistence of DHBV DNA in the liver indicated that the duration of adefovir treatment was not sufficient to clear DHBV-infected hepatocytes. Moreover, these results substantiate the necessity for maximal inhibition of viral replication to increase the initial phase of viral clearance to prevent further cycles of infection of naive hepatocytes. Since adefovir is unable to block the initial formation of CCC DNA, new hepatocytes will continue to be infected as long as residual circulating virions are present in the bloodstream. Once the clearance of free virus from plasma is achieved, the duration of adefovir therapy required to achieve viral elimination would depend only on the persistence of CCC DNA and the longevity of hepatocytes (32). It is therefore important to further evaluate combination treatments to obtain a synergistic effect on the first phase of viral clearance, as this was elegantly suggested by in vitro experiments with lamivudine, penciclovir, and adefovir (6, 7), but also with lamivudine and famciclovir in patients (27).
Persistence of virus during therapy may result from the long half-life of viral CCC DNA in the liver (49) as well as the presence of inaccessible extrahepatocytic reservoirs, such as bile duct epithelial cells, a compartment of DHBV replication previously shown to be resistant to penciclovir and lamivudine therapy but susceptible to adefovir (37). These data and our results suggest that viral clearance during nucleoside analog therapy could be enhanced by protecting uninfected cells from residual circulating virions by using additional agents, such as neutralizing antibodies (33), cytokines (16), or other pathways. In fact, adefovir was also reported to exhibit immunomodulatory effects, including stimulation of natural killer cell activity and interferon alpha production (4). Other approaches, such as combining antiviral therapy with DNA-based vaccination to induce a specific antiviral TH1 immune response (41), should be further evaluated as strategies to enhance viral clearance.
Acknowledgments
Julien Delmas and Olivier Schorr contributed equally to this work.
REFERENCES
- 1.Aguesse-Germon, S., S. H. Liu, M. Chevallier, C. Pichoud, C. Jamard, C. Borel, C. K. Chu, C. Trépo, Y.-C. Cheng, and F. Zoulim. 1998. Inhibitory effect of 2′-fluoro-5-methyl-β-l-arabinofuranosyl-uracil on duck hepatitis B virus replication. Antimicrob. Agents Chemother. 42:369-376. [DOI] [PMC free article] [PubMed]
- 2.Borel, C., O. Schorr, I. Durand, F. Zoulim, A. Kay, C. Trepo, and O. Hantz. 2001. Initial amplification of duck hepatitis B virus covalently closed circular DNA after in vitro infection of embryonic duck hepatocytes is increased by cell cycle progression. Hepatology34:168-179. [DOI] [PubMed]
- 3.Borel, C., C. Sunyach, O. Hantz, C. Trepo, and A. Kay. 1998. Phosphorylation of DHBV pre-S: identification of the major site of phosphorylation and effects of mutations on the virus life cycle. Virology 242:90-98. [DOI] [PubMed] [Google Scholar]
- 4.Calio, R., N. Villani, E. Balestra, F. Sesa, A. Holy, J. Balzarini, E. De Clercq, C. F. Perno, and V. Del Gobbo. 1994. Enhancement of natural killer activity and interferon induction by different acyclic nucleoside phosphonates. Antivir. Res. 23:77-89. [DOI] [PubMed] [Google Scholar]
- 5.Civitico, G. M., and S. A. Locarnini. 1994. The half-life of duck hepatitis B virus supercoiled DNA in congenitally infected primary hepatocyte cultures. Virology 203:81-89. [DOI] [PubMed] [Google Scholar]
- 6.Colledge, D., G. Civitico, S. Locarnini, and T. Shaw. 2000. In vitro antihepadnaviral activities of combinations of penciclovir, lamivudine, and adefovir. Antimicrob. Agents Chemother. 44:551-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colledge, D., S. Locarnini, and T. Shaw. 1997. Synergistic inhibition of hepadnaviral replication by lamivudine in combination with penciclovir in vitro. Hepatology 26:216-225. [DOI] [PubMed] [Google Scholar]
- 8.Cundy, K. C., J. A. Fishback, J. P. Shaw, M. L. Lee, K. F. Soike, G. C. Visor, and W. A. Lee. 1994. Oral bioavailability of the antiretroviral agent 9-(2-phosphonylmethoxyethyl)adenine (PMEA) from three formulations of the prodrug bis(pivaloyloxymethyl)-PMEA in fasted male cynomolgus monkeys. Pharm. Res. 11:839-843. [DOI] [PubMed] [Google Scholar]
- 9.Dandri, M., M. R. Burda, H. Will, and J. Petersen. 2000. Increased hepatocyte turnover and inhibition of woodchuck hepatitis B virus replication by adefovir in vitro do not lead to reduction of the closed circular DNA. Hepatology 32:139-146. [DOI] [PubMed] [Google Scholar]
- 10.Dienstag, J. L., E. R. Schiff, T. L. Wright, R. P. Perrillo, H. W. L. Hann, Z. Goodman, L. Crowther, L. D. Condreay, M. Woessner, M. Rubin, and N. A. Brown. 1999. Lamivudine as initial treatment for chronic hepatitis B in the United States. N. Engl. J. Med. 341:1256-1263. [DOI] [PubMed] [Google Scholar]
- 11.Doo, E., and T. J. Liang. 2001. Molecular anatomy and pathophysiologic implications of drug resistance in hepatitis B virus infection. Gastroenterology 120:1000-1008. [DOI] [PubMed] [Google Scholar]
- 12.Fautz, R., B. Hussein, and C. Hechenberger. 1991. Application of the neutral red assay (NR assay) to monolayer cultures of primary hepatocytes: rapid colorimetric viability determination for the unscheduled DNA synthesis test (UDS). Mutat. Res. 253:173-179. [DOI] [PubMed] [Google Scholar]
- 13.Fourel, I., J. M. Cullen, J. Saputelli, C. E. Aldrich, P. Schaffer, D. R. Averett, J. Pugh, and W. S. Mason. 1994. Evidence that hepatocyte turnover is required for rapid clearance of duck hepatitis B virus during antiviral therapy of chronically infected ducks. J. Virol. 68:8321-8330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu, L., and Y. C. Cheng. 2000. Characterization of novel human hepatoma cell lines with stable hepatitis B virus secretion for evaluating new compounds against lamivudine- and penciclovir-resistant virus. Antimicrob. Agents Chemother. 44:3402-3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilson, R. J., K. B. Chopra, A. M. Newell, I. M. Murray-Lyon, M. R. Nelson, S. J. Rice, R. S. Tedder, J. Toole, H. S. Jaffe, and I. V. Weller. 1999. A placebo-controlled phase I/II study of adefovir dipivoxil in patients with chronic hepatitis B virus infection. J. Viral Hepat. 6:387-395. [DOI] [PubMed] [Google Scholar]
- 16.Guidotti, L. G., R. Rochford, J. Chung, M. Shapiro, R. Purcell, and F. V. Chisari. 1999. Viral clearance without destruction of infected cells during acute HBV infection. Science 284:825-829. [DOI] [PubMed] [Google Scholar]
- 17.Guo, J. T., H. Zhou, C. Liu, C. Aldrich, J. Saputelli, T. Whitaker, M. I. Barrasa, W. S. Mason, and C. Seeger. 2000. Apoptosis and regeneration of hepatocytes during recovery from transient hepadnavirus infections. J. Virol. 74:1495-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heijtink, R. A., G. A. Dewilde, J. Kruining, L. Berk, J. Balzarini, E. Declercq, A. Holy, and S. W. Schalm. 1993. Inhibitory effect of 9-(2-phosphonylmethoxyethyl)adenine (PMEA) on human and duck hepatitis-B virus infection. Antivir. Res. 21:141-153. [DOI] [PubMed] [Google Scholar]
- 19.Heijtink, R. A., J. Kruining, G. A. Dewilde, J. Balzarini, E. Declercq, and S. W. Schalm. 1994. Inhibitory effects of acyclic nucleoside phosphonates on human hepatitis B virus and duck hepatitis B virus infections in tissue culture. Antimicrob. Agents Chemother. 38:2180-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoofnagle, J. H., and A. M. DiBisceglie. 1997. Drug therapy: the treatment of chronic viral hepatitis. N. Engl. J. Med. 336:347-356. [DOI] [PubMed] [Google Scholar]
- 21.Jilbert, A. R., T. T. Wu, J. M. England, P. M. Hall, N. Z. Carp, A. P. O'Connell, and W. S. Mason. 1992. Rapid resolution of duck hepatitis B virus infections occurs after massive hepatocellular involvement. J. Virol. 66:1377-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kann, M., A. Bischof, and W. H. Gerlich. 1997. In vitro model for the nuclear transport of the hepadnavirus genome. J. Virol. 71:1310-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kock, J., and H. J. Schlicht. 1993. Analysis of the earliest steps of hepadnavirus replication: genome repair after infectious entry into hepatocytes does not depend on viral polymerase activity. J. Virol. 67:4867-4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kruining, J., R. A. Heijtink, and S. W. Schalm. 1995. Antiviral agents in hepatitis B virus transfected cell lines: inhibitory and cytotoxic effect related to time of treatment. J. Hepatol. 22:263-267. [DOI] [PubMed] [Google Scholar]
- 25.Lai, C. L., R. N. Chien, N. W. Y. Leung, T. T. Chang, R. Guan, D. I. Tai, K. Y. Ng, P. C. Wu, J. C. Dent, J. Barber, S. L. Stephenson, and D. F. Gray. 1998. A one-year trial of lamivudine for chronic hepatitis B. N. Engl. J. Med. 339:61-68. [DOI] [PubMed] [Google Scholar]
- 26.Lambert, V., D. Fernholz, R. Sprengel, I. Fourel, G. Deleage, G. Wildner, C. Peyret, C. Trepo, L. Cova, and H. Will. 1990. Virus-neutralizing monoclonal antibody to a conserved epitope on the duck hepatitis B virus pre-S protein. J. Virol. 64:1290-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lau, G. K., M. Tsiang, J. Hou, S. Yuen, W. F. Carman, L. Zhang, C. S. Gibbs, and S. Lam. 2000. Combination therapy with lamivudine and famciclovir for chronic hepatitis B-infected Chinese patients: a viral dynamics study. Hepatology 32:394-399. [DOI] [PubMed] [Google Scholar]
- 28.Lee, W. M. 1997. Hepatitis B virus infection. N. Engl. J. Med. 337:1733-1745. [DOI] [PubMed] [Google Scholar]
- 29.LeGuerhier, F., C. Pichoud, S. Guerret, M. Chevallier, C. Jamard, O. Hantz, X. Y. Li, S. H. Chen, I. King, C. Trepo, Y. C. Cheng, and F. Zoulim. 2000. Characterization of the antiviral effect of 2′,3′-dideoxy-2′,3′-didehydro-beta-L-5-fluorocytidine in the duck hepatitis B virus infection model. Antimicrob. Agents Chemother. 44:111-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Locarnini, S., and C. Birch. 1999. Antiviral chemotherapy for chronic hepatitis B infection: lessons learned from treating HIV-infected patients. J. Hepatol. 30:536-550. [DOI] [PubMed] [Google Scholar]
- 31.Luscombe, C., J. Pedersen, E. Uren, and S. Locarnini. 1996. Long-term ganciclovir chemotherapy for congenital duck hepatitis B virus infection in vivo: effect on intrahepatic-viral DNA, RNA, and protein expression. Hepatology 24:766-773. [DOI] [PubMed] [Google Scholar]
- 32.Mason, W. S., J. Cullen, G. Moraleda, J. Saputelli, C. E. Aldrich, D. S. Miller, B. Tennant, L. Frick, D. Averett, L. D. Condreay, and A. R. Jilbert. 1998. Lamivudine therapy of WHV-infected woodchucks. Virology 245:18-32. [DOI] [PubMed] [Google Scholar]
- 33.Mason, W. S., J. Cullen, J. Saputelli, T. T. Wu, C. Liu, W. T. London, E. Lustbader, P. Schaffer, A. P. Oconnell, I. Fourel, C. E. Aldrich, and A. R. Jilbert. 1994. Characterization of the antiviral effects of 2′ carbodeoxyguanosine in ducks chronically infected with duck hepatitis B virus. Hepatology 19:398-411. [PubMed] [Google Scholar]
- 34.Moraleda, G., J. Saputelli, C. E. Adrich, D. Averett, L. Condreay, and W. S. Mason. 1997. Lack of effect of antiviral therapy in nondividing hepatocyte cultures on the closed circular DNA of woodchuck hepatitis virus. J. Virol. 71:9392-9399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nafa, S., S. Ahmed, D. Tavan, C. Pichoud, F. Berby, L. Stuyver, M. Johnson, P. Merle, H. Abidi, C. Trepo, and F. Zoulim. 2000. Early detection of viral resistance by determination of hepatitis B virus polymerase mutations in patients treated by lamivudine for chronic hepatitis B. Hepatology 32:1078-1088. [DOI] [PubMed] [Google Scholar]
- 36.Newbold, J., H. Xin, M. Teneza, G. Sherman, J. Dean, S. Bowden, and S. Locarnini. 1995. The covalently closed duplex form of the hepadnavirus genome exists in situ as a heterogeneous population of viral minichromosomes. J. Virol. 69:3350-3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nicoll, A. J., D. L. Colledge, J. J. Toole, P. W. Angus, R. A. Smallwood, and S. A. Locarnini. 1998. Inhibition of duck hepatitis B virus replication by 9-(2-phosphonylmethoxyethyl)adenine, an acyclic phosphonate nucleoside analogue. Antimicrob. Agents Chemother. 42:3130-3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nowak, M. A., S. Bonhoeffer, A. M. Hill, R. Boehme, H. C. Thomas, and H. Mcdade. 1996. Viral dynamics in hepatitis B virus infection. Proc. Natl. Acad. Sci. USA 93:4398-4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ono, S. K., N. Kato, Y. Shiratori, J. Kato, T. Goto, R. F. Schinazi, F. J. Carrilho, and M. Omata. 2001. The polymerase L528M mutation cooperates with nucleotide binding-site mutations, increasing hepatitis B virus replication and drug resistance. J. Clin. Investig. 107:449-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perrillo, R., E. Schiff, E. Yoshida, A. Statler, K. Hirsch, T. Wright, K. Gutfreund, P. Lamy, and A. Murray. 2000. Adefovir dipivoxil for the treatment of lamivudine-resistant hepatitis B mutants. Hepatology 32:129-134. [DOI] [PubMed] [Google Scholar]
- 41.Rollier, C., C. Sunyach, L. Barraud, N. Madani, C. Jamard, C. Trepo, and L. Cova. 1999. Protective and therapeutic effect of DNA-based immunization against hepadnavirus large envelope protein. Gastroenterology 116:658-665. [DOI] [PubMed] [Google Scholar]
- 42.Seigneres, B., S. Aguesse-Germon, C. Pichoud, I. Vuillermoz, C. Jamard, C. Trepo, and F. Zoulim. 2001. Duck hepatitis B virus polymerase gene mutants associated with resistance to lamivudine have a decreased replication capacity in vitro and in vivo. J. Hepatol. 34:114-122. [DOI] [PubMed] [Google Scholar]
- 43.Summers, J., P. M. Smith, and A. L. Horwich. 1990. Hepadnavirus envelope proteins regulate covalently closed circular DNA amplification. J. Virol. 64:2819-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsiang, M., J. F. Rooney, J. J. Toole, and C. S. Gibbs. 1999. Biphasic clearance kinetics of hepatitis B virus from patients during adefovir dipivoxil therapy. Hepatology 29:1863-1869. [DOI] [PubMed] [Google Scholar]
- 45.Turin, F., C. Borel, M. Benchaib, T. Kay, C. Jamard, C. Guguen-guillouzo, C. Trepo, and O. Hantz. 1996. n-Butyrate, a cell cycle blocker, inhibits early amplification of duck hepatitis B virus covalently closed circular DNA after in vitro infection of duck hepatocytes. J. Virol. 70:2691-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tuttleman, J. S., C. Pourcel, and J. W. Summers. 1986. Formation of the pool of covalently closed circular viral DNA in hepadnavirus-infected cells. Cell 47:451-460. [DOI] [PubMed] [Google Scholar]
- 47.Wu, T. T., L. Coates, L. Aldrich, J. Summers, and W. S. Mason. 1990. In hepatocytes infected with duck hepatitis B virus, the template for viral RNA synthesis is amplified by an intracellular pathway. Virology 175:255-261. [DOI] [PubMed] [Google Scholar]
- 48.Xiong, X. F., C. Flores, H. Yang, J. J. Toole, and C. S. Gibbs. 1998. Mutations in hepatitis B DNA polymerase associated with resistance to lamivudine do not confer resistance to adefovir in vitro. Hepatology 28:1669-1673. [DOI] [PubMed] [Google Scholar]
- 49.Zhu, Y., T. Yamamoto, J. Cullen, J. Saputelli, C. E. Aldrich, D. S. Miller, S. Litwin, P. A. Furman, A. R. Jilbert, and W. S. Mason. 2001. Kinetics of hepadnavirus loss from the liver during inhibition of viral DNA synthesis. J. Virol. 75:311-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zoulim, F., and C. Trepo. 1998. Drug therapy for chronic hepatitis B: antiviral efficacy and influence of hepatitis B virus polymerase mutations on the outcome of therapy. J. Hepatol. 29:151-168. [DOI] [PubMed] [Google Scholar]