Abstract
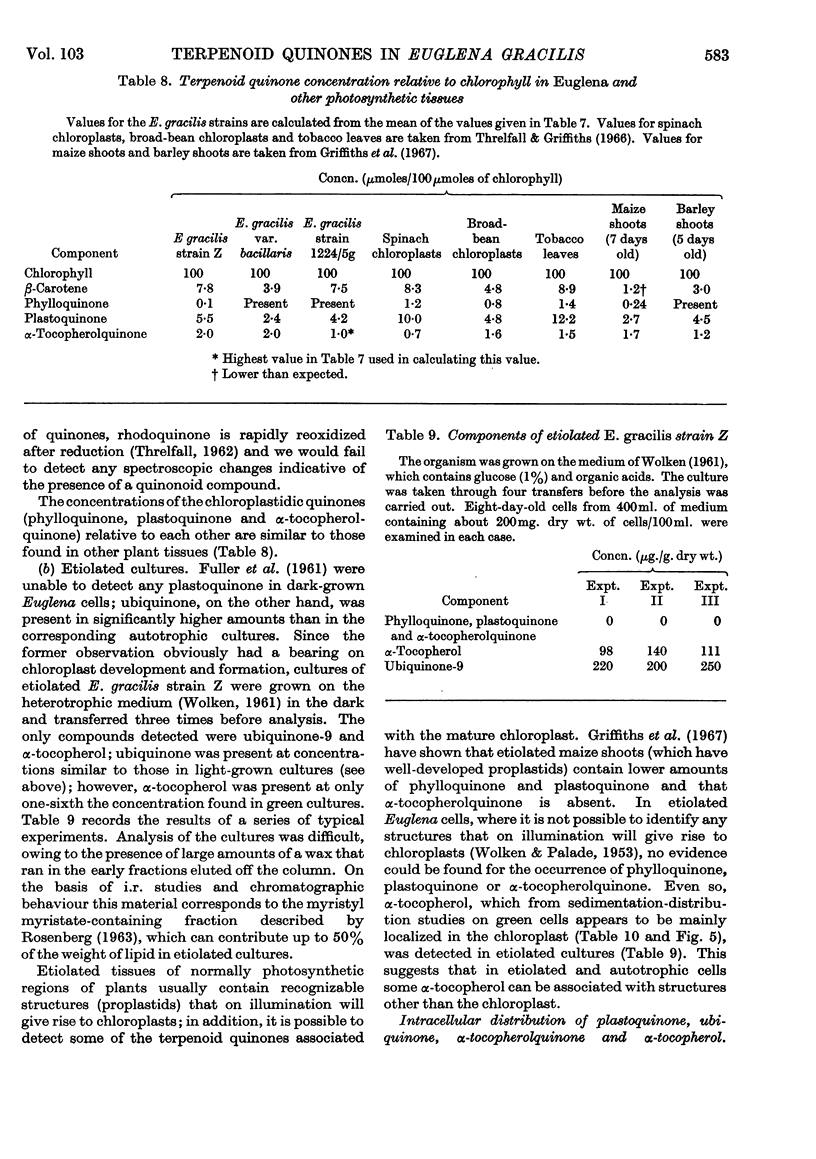
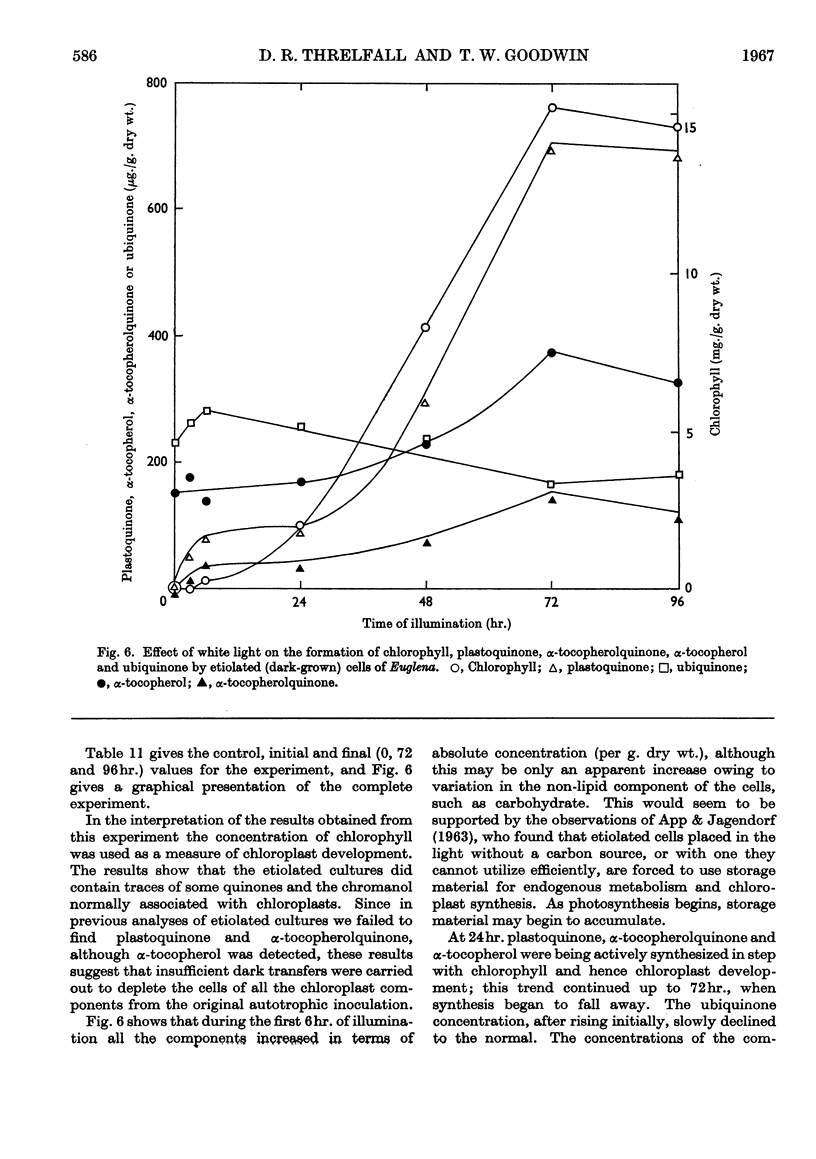
1. Light-grown cells of Euglena gracilis strain Z, var. bacillaris and 1224/5g contain phylloquinone, plastoquinone, α-tocopherol, α-tocopherolquinone and ubiquinone-9 (i.e. ubiquinone with 9 isoprene units/mol.). 2. The concentration (per g. dry wt.) of plastoquinone (and chlorophyll) in light-grown cells of strain Z was governed by the composition of the culture medium and age of the cells. Highest yields of plastoquinone were obtained under autotrophic conditions, the concentration reaching a maximum after 6–8 days' growth. The concentrations were less in heterotrophic media. The concentration of ubiquinone was relatively unaffected by the age of the cells or composition of the medium. 3. In light-grown cells of strain Z plastoquinone, α-tocopherolquinone and α-tocopherol were mainly localized in the chloroplast; ubiquinone was found to be in the mitochondria. 4. Etiolated (dark-grown) cells of strain Z contained no phylloquinone, plastoquinone or α-tocopherolquinone; α-tocopherol was present in lower concentrations compared with light-grown cells; ubiquinone concentrations were similar to those for light-grown cells. The presence of α-tocopherol in etiolated cells suggested that this chromanol was not entirely confined to the chloroplast. 5. On illumination of etiolated cells of strain Z the chloroplastidic components plastoquinone, α-tocopherolquinone and α-tocopherol were synthesized in step with chloroplast formation. Ubiquinone concentrations, as expected, were unaffected. 6. [2-14C]Mevalonic acid, the specific distal terpenoid precursor, was not incorporated into any of the terpenoid components examined. This was attributed to the impermeability of the cell wall to this compound, rather than to a novel pathway of terpenoid biosynthesis.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRAWERMAN G., CHARGAFF E. Changes in protein and ribonucleic acid during the formation of chlorplasts in Euglena gracilis. Biochim Biophys Acta. 1959 Jan;31(1):164–171. doi: 10.1016/0006-3002(59)90452-4. [DOI] [PubMed] [Google Scholar]
- Crane F. L. Isolation of Two Quinones with Coenzyme Q Activity from Alfalfa. Plant Physiol. 1959 Sep;34(5):546–551. doi: 10.1104/pp.34.5.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DILLEY R. A., CRANE F. L. A specific assay for tocopherols in plant tissue. Anal Biochem. 1963 Jun;5:531–541. doi: 10.1016/0003-2697(63)90073-3. [DOI] [PubMed] [Google Scholar]
- Dam H., Glavind J. Vitamin K in the plant. Biochem J. 1938 Mar;32(3):485–487. doi: 10.1042/bj0320485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das B. C., Lounasmaa M., Tendille C., Lederer E. Mass spectrometry of plastoquinones. The structure of the plastoquinones B,C and D. Biochem Biophys Res Commun. 1965 Nov 22;21(4):318–322. doi: 10.1016/0006-291x(65)90195-6. [DOI] [PubMed] [Google Scholar]
- FULLER R. C., SMILLIE R. M., RIGOPOULOS N., YOUNT V. Comparative studies of some quinones in photosynthetic systems. Arch Biochem Biophys. 1961 Nov;95:197–202. doi: 10.1016/0003-9861(61)90135-7. [DOI] [PubMed] [Google Scholar]
- GREEN J., PRICE S. A., GARE L. Tocopherols in micro-organisms. Nature. 1959 Oct 24;184(Suppl 17):1339–1339. doi: 10.1038/1841339a0. [DOI] [PubMed] [Google Scholar]
- Griffiths W. T., Threlfall D. R., Goodwin T. W. Nature, intracellular distribution and formation of terpenoid quinones in maize and barley shoots. Biochem J. 1967 May;103(2):589–600. doi: 10.1042/bj1030589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENNINGER M. D., DILLEY R. A., CRANE F. L. Restoration of ferricyanide reduction in acetone-extracted chloroplasts by beta and gamma tocopherol quinones. Biochem Biophys Res Commun. 1963 Feb 6;10:237–242. doi: 10.1016/0006-291x(63)90423-6. [DOI] [PubMed] [Google Scholar]
- KEGEL L. P., HENNINGER M. D., CRANE F. L. Two new quinones from chloroplasts. Biochem Biophys Res Commun. 1962 Jul 19;8:294–298. doi: 10.1016/0006-291x(62)90281-4. [DOI] [PubMed] [Google Scholar]
- LAWSON D. E., THRELFALL D. R., GLOVER J., MORTON R. A. Biosynthesis of ubiquinone in the rat. Biochem J. 1961 Apr;79:201–208. doi: 10.1042/bj0790201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LESTER R. L., CRANE F. L. The natural occurrence of coenzyme Q and related compounds. J Biol Chem. 1959 Aug;234(8):2169–2175. [PubMed] [Google Scholar]
- MCKENNA M., HENNINGER M. D., CRANE F. L. A SECOND NAPHTHOQUINONE IN SPINACH CHLOROPLASTS. Nature. 1964 Aug 1;203:524–525. doi: 10.1038/203524a0. [DOI] [PubMed] [Google Scholar]
- PAGE A. C., Jr, GALE P. H., KONIUSZY F., FOLKERS K. Coenzyme Q. IX. Coenzyme Q9 and Q10 content of dietary components. Arch Biochem Biophys. 1959 Dec;85:474–477. doi: 10.1016/0003-9861(59)90513-2. [DOI] [PubMed] [Google Scholar]
- Pennock J. F., Neiss G., Mahler H. R. Biochemical studies on the developing avian embryo. 5. Ubiquinone and some other unsaponifiable lipids. Biochem J. 1962 Dec;85(3):530–537. doi: 10.1042/bj0850530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSENBERG A. A COMPARISON OF LIPID PATTERNS IN PHOTOSYNTHESIZING AND NONPHOTOSYNTHESIZING CELLS OF EUGLENA GRACILIS. Biochemistry. 1963 Sep-Oct;2:1148–1154. doi: 10.1021/bi00905a042. [DOI] [PubMed] [Google Scholar]
- THRELFALL D. R., GOODWIN T. W. UBIQUINONE-50 AND PLASTOQUINONE-45 IN PLANT TISSUE CULTURES OF PAUL'S SCARLET ROSE. Biochim Biophys Acta. 1963 Nov 15;78:532–533. doi: 10.1016/0006-3002(63)90916-8. [DOI] [PubMed] [Google Scholar]
- Threlfall D. R., Griffiths W. T., Goodwin T. W. Isolation of two analogues of plastoquinone from senescent leaves of tobacco. Biochim Biophys Acta. 1965 Jul 22;102(2):614–618. doi: 10.1016/0926-6585(65)90152-4. [DOI] [PubMed] [Google Scholar]
- WOLKEN J. J., PALADE G. E. An electron microscope study of two flagellates, chloroplast structure and variation. Ann N Y Acad Sci. 1953 Oct 14;56(5):873–889. doi: 10.1111/j.1749-6632.1953.tb30266.x. [DOI] [PubMed] [Google Scholar]
- Whittle K. J., Dunphy P. J., Pennock J. F. The isolation and properties of delta-tocotrienol from Hevea latex. Biochem J. 1966 Jul;100(1):138–145. doi: 10.1042/bj1000138. [DOI] [PMC free article] [PubMed] [Google Scholar]


