Abstract
Amphotericin B, flucytosine, fluconazole, and voriconazole alone and in combination were evaluated against isolates of Candida lusitaniae. MICs were determined by broth microdilution and Etest, and time-kill studies were conducted. Amphotericin B resulted in fungicidal activity against most isolates, whereas fluconazole, voriconazole, and flucytosine produced primarily fungistatic activities. The addition of flucytosine to amphotericin B resulted in a faster rate and greater extent of kill for isolates for which the MICs of amphotericin B were elevated.
Candida lusitaniae was first isolated from the digestive tracts of warm-blooded animals. C. lusitaniae is rarely an opportunistic human pathogen; however, when implicated it often causes serious and fatal disease (1). Despite its low prevalence, C. lusitaniae is classified as an emerging opportunistic pathogen (3). A hallmark of C. lusitaniae is its innate resistance or rapid development of resistance to amphotericin B (4). Pfaller and colleagues demonstrated the ability of a single strain to develop resistance to amphotericin B rapidly during antifungal therapy (8).
As a result of the perceived high level of resistance of C. lusitaniae to amphotericin B and the relative lack of information on this topic, we evaluated the antifungal activities of amphotericin B, fluconazole, voriconazole, and flucytosine alone and in combination against C. lusitaniae.
Antifungal agents.
Amphotericin B (Sigma Chemical Company, St. Louis, Mo.), flucytosine (Sigma), fluconazole (Pfizer, Inc., New York, N.Y.), and voriconazole (Pfizer) were utilized for susceptibility and time-kill procedures. A stock solution of each antifungal agent was prepared utilizing RPMI 1640 medium (Sigma) buffered to pH 7.0 with 0.165 M morpholinepropanesulfonic acid (MOPS) buffer (Sigma) as the diluent. Dimethyl sulfoxide (DMSO) was used to aid the solubilization of amphotericin B and voriconazole. The final concentration of DMSO was such that its concentration in the test solutions comprised less than 1% of the total solution composition. Growth curves were conducted with DMSO at concentrations equal to those present in the test solutions to verify the lack of an inhibitory effect on the growth of the test isolates. Stock solutions were stored at −70°C until needed for testing.
Test isolates.
Eleven clinical isolates of C. lusitaniae were obtained for testing. Five of the test isolates were obtained from the organism collection of the Special Microbiology Laboratory, Department of Pathology, University of Iowa College of Medicine (200015.0910, 20090.0370, 20091.0820, 20015.0900, and OY14084). Five test isolates were obtained from the Fungus Testing Laboratory, San Antonio, Tex. (FTL-1, FTL-3, FTL-4, FTL-5, FTL-6). The final test isolate was acquired from the Mycology Research Laboratories at the University of Houston/University of Texas M. D. Anderson Cancer Center (RL-1).
Antifungal susceptibility testing.
The MICs of amphotericin B, flucytosine, fluconazole, and voriconazole for each test isolate were determined by broth microdilution according to the methods approved by the National Committee for Clinical Laboratory Standards (M27-A) and by Etest according to the manufacturer's specifications (AB Biodisk, Solna, Sweden) (7). MICs of voriconazole were not determined by Etest.
Time-kill curve procedures.
Time-kill procedures were conducted as described previously (2, 5). Fungi were obtained from stored samples and subcultured twice on potato dextrose agar plates (Remel) prior to testing. Fungal suspensions were prepared in sterile water by touching three to five colonies from a 24- to 48-h-old culture plate and adjusting the resulting suspension to a 0.5 McFarland turbidity standard (approximately 1 × 106 to 5 × 106 CFU/ml) by using spectrophotometric methods. One milliliter of the fungal suspension was added to 9 ml of RPMI 1640 buffered with MOPS to pH 7.0 with or without drug, providing the starting inoculum of approximately 1 × 105 to 5 × 105 CFU/ml. The antifungal concentrations tested alone and in combination for each isolate were 2, 75, 40, and 4 μg of amphotericin B, flucytosine, fluconazole, and voriconazole per ml, respectively. These concentrations were selected to represent the peak concentrations achieved in the serum of ill patients (10, 11). For combinations including fluconazole or voriconazole, yeast was exposed to the azole for 12 h at 35°C prior to the addition of amphotericin B or flucytosine. To remove the azole antifungal agent, following the preexposure period, the culture vials were centrifuged for 8 min at 1,400 × g, the supernatant was removed, and the resulting cell pellet was readjusted with sterile saline to a 0.5 McFarland turbidity standard. One milliliter of the fungal suspension was added to 9 ml of RPMI buffered with MOPS, providing a starting inoculum of 1 × 105 to 5 × 105 CFU/ml. Finally, the azole and amphotericin B or flucytosine were added again to the test suspension. The culture vials were incubated with agitation at 35°C. At predetermined time points (0, 2, 4, 6, 8, and 24 h following the addition of the antifungal agent), a 0.1-ml sample was removed from each culture vial and serially diluted 1:10 in sterile water, and a 30-μl aliquot was plated on potato dextrose agar (Remel). Colony counts were determined after incubation of the plates at 35°C for 24 to 48 h. When colony counts were suspected to be less than 1,000 CFU/ml, 30-μl samples were removed from the culture vial and plated without dilution. The limit of quantification by these methods is 100 CFU/ml. Kill curve experiments were conducted in duplicate.
Analysis.
Mean colony count data (log10 of the number of CFU per milliliter) from kill curve replicates were plotted versus time and used for visual comparisons of the rate and extent of antifungal activity. Fungicidal activity was defined as a ≥3 log10 (99.9%) reduction in CFU/ml from the starting inoculum. Fungistatic activity was a <99.9% reduction in CFU/ml from the starting inoculum. The relationship between activity measured by microdilution, Etest, and time to achieve a fungicidal endpoint was plotted using linear regression.
Antifungal susceptibility results.
Median susceptibility test results are presented in Table 1. The ranges of the MICs were 0.25 to 2 μg of amphotericin B/ml, 0.06 to 128 μg of flucytosine/ml, 0.12 to 2 μg of fluconazole/ml, and 0.007 to 0.06 μg of voriconazole/ml, as determined by broth microdilution. Etest results ranged from 0.38 to 32, 0.008 to 32, and 0.064 to 1 μg of amphotericin B, flucytosine, and fluconazole per ml, respectively.
TABLE 1.
Susceptibility testing results for amphotericin B, 5-flucytosine, fluconazole, and voriconazole by microdilution and Etest
| Isolate | MIC (μg/ml) of:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Amphotericin B
|
5-Flucytosine
|
Fluconazole
|
Voriconazole
|
||||||
| MDa | Etest | MD | Etest | MD | Etest | MD | Etest | ||
| FTL-1 | 2.0 | 8.0 | 0.06 | 0.008 | 0.12 | 0.064 | 0.007 | —b | |
| FTL-3 | 2.0 | 32.0 | 0.06 | 0.003 | 0.50 | 0.125 | 0.03 | — | |
| FTL-4 | 1.0 | 3.0 | 0.06 | 0.032 | 0.25 | 0.19 | 0.015 | — | |
| FTL-5 | 1.0 | 4.0 | 0.06 | 0.023 | 0.12 | 0.32 | 0.007 | — | |
| FTL-6 | 1.0 | 0.50 | 0.06 | 0.047 | 0.25 | 0.125 | 0.03 | — | |
| RL-1 | 1.0 | 0.38 | 0.06 | 0.047 | 2.0 | 1.0 | 0.007 | — | |
| 20015.0910 | 1.0 | 0.38 | 0.06 | 0.012 | 0.25 | 0.38 | 0.06 | — | |
| 20090.0370 | 0.25 | 0.125 | 0.06 | 0.012 | 0.50 | 0.75 | 0.007 | — | |
| 20091.0820 | 0.50 | 0.38 | 0.06 | 0.64 | 1.0 | 0.75 | 0.007 | — | |
| 20015.0900 | 1.0 | 2.0 | 0.06 | 0.032 | 0.25 | 0.125 | 0.015 | — | |
| OY14084 | 1.0 | 0.75 | 128.0 | 32.0 | 0.50 | 0.125 | 0.007 | — | |
MD, microdilution. —, MICs of voriconazole were not determined by Etest.
Time-kill curves.
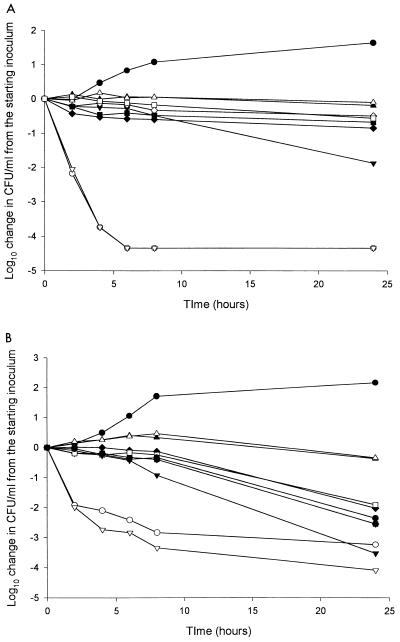
Figure 1shows representative kill curves for the antifungal agents tested alone and in combination. Table 2 shows the log10 change in CFU per milliliter for all of the isolates with each drug alone. Amphotericin B produced fungicidal activity against 10 of 11 isolates. Flucytosine resulted in fungicidal activity against 2 of the 11 isolates tested at 24 h. One isolate exhibited growth from the starting inoculum following exposure to flucytosine (OY14084). However, amphotericin B, fluconazole, and voriconazole exhibited fungicidal activity against the same isolate, causing nearly a 4 log10 decrease in CFU/ml from the starting inoculum. Fluconazole produced fungicidal activity against 2 of 11 C. lusitaniae isolates and fungistatic activity against 6 of 11 isolates. Growth, or an increase in CFU per milliliter from the starting inoculum, was noted with three isolates when tested with fluconazole alone. Voriconazole produced primarily fungistatic effects, occurring with 8 of 11 isolates. Against one isolate, voriconazole produced fungicidal effects, whereas growth occurred for two isolates.
FIG. 1.
Representative time-kill curve plots for amphotericin B-susceptible C. lusitaniae isolate 20091.082 (A) and amphotericin B-resistant C. lusitaniae isolate FTL-1 (B) with antifungal agents. •, control; ○, amphotericin B; ▴, fluconazole; ▵, voriconazole; ▾, flucytosine; ▿, amphotericin B and flucytosine; ♦, fluconazole and amphotericin B; ⋄, fluconazole and flucytosine; ▪, voriconazole and amphotericin B; □, voriconazole and flucytosine.
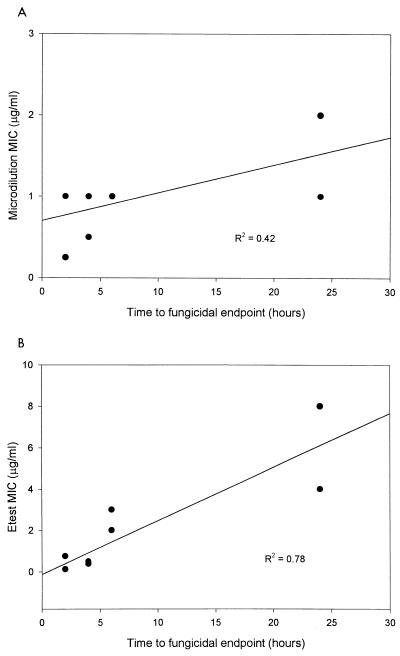
For amphotericin B the time necessary to achieve a fungicidal endpoint ranged from 2 to >24 h. Correlations of the time necessary to reach a fungicidal endpoint for amphotericin B with the MIC determined by broth microdilution (R2 = 0.42) and Etest (R2 = 0.78) are presented in Fig. 2.
FIG. 2.
Relationship between amphotericin B and rate of fungicidal activity with the MIC determined by microdilution (A) and Etest (B).
The addition of flucytosine to amphotericin B resulted in a faster rate of kill, measured by the time necessary to achieve a fungicidal endpoint, for isolates for which amphotericin B MICs were elevated, as measured by Etest. For isolates for which amphotericin B MICs were ≥3 μg/ml by Etest, there was a trend toward a shorter time to the fungicidal endpoint, 9.3 versus 14.5 h, for the combination of amphotericin B plus flucytosine compared to amphotericin B alone (P = 0.068) by the Wilcoxon signed rank test. Three of the four isolates for which amphotericin B MICs were elevated also showed a greater extent of activity at 24 h, with an average of 0.5 log10 greater reduction with the combination. Other combinations did not produce improvement in activity versus single agents. The addition of an azole antifungal agent to amphotericin B resulted in a decrease in killing by amphotericin B. The combination of an azole antifungal agent and flucytosine resulted in a slight decrease in the activity of flucytosine (Fig. 1).
Amphotericin B was the antifungal agent that exhibited the greatest activity against the isolates tested despite the fact that C. lusitaniae is known for displaying resistance to amphotericin B. Amphotericin B displayed fungicidal activity against all but one isolate tested. The one isolate against which amphotericin B did not display fungicidal activity (FLT-3) was the isolate for which the amphotericin B MIC was the highest, as tested by both Etest and broth microdilution. While a fungicidal endpoint was not reached, amphotericin B did result in a 2.95 log10 reduction in CFU from the starting inoculum. The rate of kill for this isolate was gradual. Many isolates exhibited a rapid decrease in CFU per milliliter within the first 4 h with little or no killing after that point (Fig. 1). In contrast, FLT-3 demonstrated a slow and steady rate of kill. It is possible that if our sampling time was extended beyond 24 h, the fungicidal endpoint may be achieved. These findings may indicate that treatment of C. lusitaniae with amphotericin B may be efficacious regardless of its MIC for the organism. However, when the MIC for the organism is elevated, the response to therapy may be relatively slow.
The rate of killing in our study correlated with the MIC for the organism tested. MICs obtained by Etest demonstrated a better correlation with the rate of killing than those obtained by broth microdilution. This may suggest that the MIC obtained by Etest is more indicative of the activity of amphotericin B. Previous reports have highlighted the detection of amphotericin B resistant isolates using NCCLS methodology. Rex and colleagues found that NCCLS M27-P methodology had a limited ability to identify amphotericin B-resistant Candida isolates (9). Wanger and colleagues tested the MICs for several C. lusitaniae isolates with macrobroth and Etest. They found that Etest often gave strikingly higher MICs than NCCLS macrobroth techniques and reported that Etest had a greater ability to discriminate between putatively amphotericin B-susceptible and -resistant isolates (12). In our study the identification of higher MICs via Etest resulted in a better correlation with activity described as the time necessary to achieve a fungicidal endpoint.
Flucytosine, when tested alone, displayed fungicidal or fungistatic activity against the majority of the isolates tested; however, the addition of flucytosine to amphotericin B increased the rate and extent of killing for isolates for which the amphotericin B MICs were elevated. Furthermore, isolates for which the amphotericin B MICs were low displayed indifference to the addition of flucytosine. These results suggest that the addition of flucytosine to amphotericin B may enhance its activity against isolates for which the MICs are elevated without compromising amphotericin B activity in more susceptible isolates. This information may be particularly useful since antifungal susceptibility testing is not universally performed.
The azole antifungal agents produced primarily fungistatic effects, with a limited number of isolates displaying growth, which was at least 0.5 log10 CFU/ml lower than the growth observed in the untreated control group. Exposure of yeast to an azole antifungal agent prior to the addition of amphotericin B resulted in decreased amphotericin B activity, as has been described previously (2, 6).
In conclusion, amphotericin B demonstrates considerable in vitro activity against C. lusitaniae, including isolates for which the MICs are elevated. This activity may be enhanced by the addition of flucytosine. The rate of kill is associated with the MIC. These data suggest that amphotericin B may be useful for the treatment of infections caused by C. lusitaniae. The relatively slow activity observed with amphotericin B against isolates for which the MICs were elevated suggests that clinical response may also be slow. The addition of flucytosine may enhance the rate and extent of killing by amphotericin B, particularly in the situation of elevated MICs of amphotericin B. The azole antifungal agents display in vitro activity against C. lusitaniae, which appears to be similar to other Candida species, which is primarily fungistatic. Animal and clinical studies are necessary to define the roles of amphotericin B, flucytosine, and the azole antifungal agents in the treatment of infections caused by C. lusitaniae.
TABLE 2.
Antifungal activities of amphotericin B, 5-flucytosine, fluconazole, and voricanazole against 11 isolates of C. lusitaniae
| Isolate | Antifungal activity ofa:
|
|||
|---|---|---|---|---|
| Amphotericin B | 5-Flucytosine | Fluconazole | Voriconazole | |
| FTL-1 | −3.2400 | −3.5300 | −0.0369 | −0.3360 |
| FTL-3 | −2.9500 | −1.8100 | −0.8940 | −0.9080 |
| FTL-4 | −4.0100 | −0.3460 | 1.1300 | 1.2600 |
| FTL-5 | −3.4500 | −2.8400 | 0.0309 | −0.3490 |
| FTL-6 | −3.1900 | −2.7400 | 0.8320 | 0.8010 |
| RL-1 | −4.1300 | −1.1500 | −0.8110 | −0.5450 |
| 20015.091 | −4.0200 | −2.9300 | −3.0500 | −2.4200 |
| 20090.037 | −4.1200 | −3.4100 | −1.0300 | −1.1500 |
| 20091.082 | −4.3400 | −1.8700 | −0.1820 | −0.0923 |
| 20015.090 | −4.1000 | −2.7200 | −1.4100 | −1.3200 |
| OY14084 | −4.0436 | 0.8789 | −4.0436 | −4.0400 |
Values are reductions in colony counts in log10 CFU per milliliter at 24 h.
Acknowledgments
This work was supported by a grant from Pfizer, Inc.
REFERENCES
- 1.Blinkhorn, R. J., D. Adelstein, and P. J. Spagnuolo. 1989. Emergence of a new opportunistic pathogen, Candida lusitaniae. J. Clin. Microbiol. 27:236-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ernst, E. J., M. E. Klepser, and M. A. Pfaller. 1998. In vitro interaction of fluconazole and amphotericin B administered sequentially against Candida albicans: effect of concentration and exposure time. Diagn. Microbiol. Infect. Dis. 32:205-210. [DOI] [PubMed] [Google Scholar]
- 3.Favel, A., A. Michel-Nguyen, C. Chastin, F. Trousson, A. Penaud, and P. Regli. 1997. In-vitro susceptibility pattern of Candida lusitaniae and evaluation of the Etest method. J. Antimicrob. Chemother. 39:591-596. [DOI] [PubMed] [Google Scholar]
- 4.Hadfield, T. L., M. B. Smith, R. E. Winn, M. G. Rinaldi, and C. Guerra. 1987. Mycoses caused by Candida lusitaniae. Rev. Infect. Dis. 9:1006-1012. [DOI] [PubMed] [Google Scholar]
- 5.Klepser, M. E., E. J. Wolfe, R. N. Jones, C. H. Nightingale, and M. A. Pfaller. 1997. Antifungal pharmacodynamic characteristics of fluconazole and amphotericin B tested against Candida albicans. Antimicrob. Agents Chemother. 41:1392-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewis, R. E., B. C. Lund, M. E. Klepser, E. J. Ernst, and M. A. Pfaller. 1998. Assessment of antifungal activities of fluconazole and amphotericin B administered alone and in combination against Candida albicans by using a dynamic in vitro mycotic infection model. Antimicrob. Agents Chemother. 42:1382-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Committee for Clinical Laboratory Standards. 1997. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 8.Pfaller, M. A., S. A. Messer, and R. J. Hollis. 1994. Strain delineation and antifungal susceptibilities of epidemiologically related and unrelated isolates of Candida lusitaniae. Diagn. Microbiol. Infect. Dis. 20:127-133. [DOI] [PubMed] [Google Scholar]
- 9.Rex, J. H., C. R. Cooper, Jr., W. G. Merz, J. N. Galgiani, and E. J. Anaissie. 1995. Detection of amphotericin B-resistant Candida isolates in a broth-based system. Antimicrob. Agents Chemother. 39:906-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwartz, S., D. Milatovic, and E. Thiel. 1997. Successful treatment of cerebral aspergillosis with a novel triazole (voriconazole) in a patient with acute leukaemia. Br. J. Haematol. 97:663-665. [DOI] [PubMed] [Google Scholar]
- 11.Summers, K. K., T. C. Hardin, S. J. Gore, and J. R. Graybill. 1997. Therapeutic drug monitoring of systemic antifungal therapy. J. Antimicrob. Chemother. 40:753-764. [DOI] [PubMed] [Google Scholar]
- 12.Wanger, A., K. Mills, P. W. Nelson, and J. H. Rex. 1995. Comparison of Etest and National Committee for Clinical Laboratory Standards broth macrodilution method for antifungal susceptibility testing: enhanced ability to detect amphotericin B-resistant Candida isolates. Antimicrob. Agents Chemother. 39:2520-2522. [DOI] [PMC free article] [PubMed] [Google Scholar]