Abstract
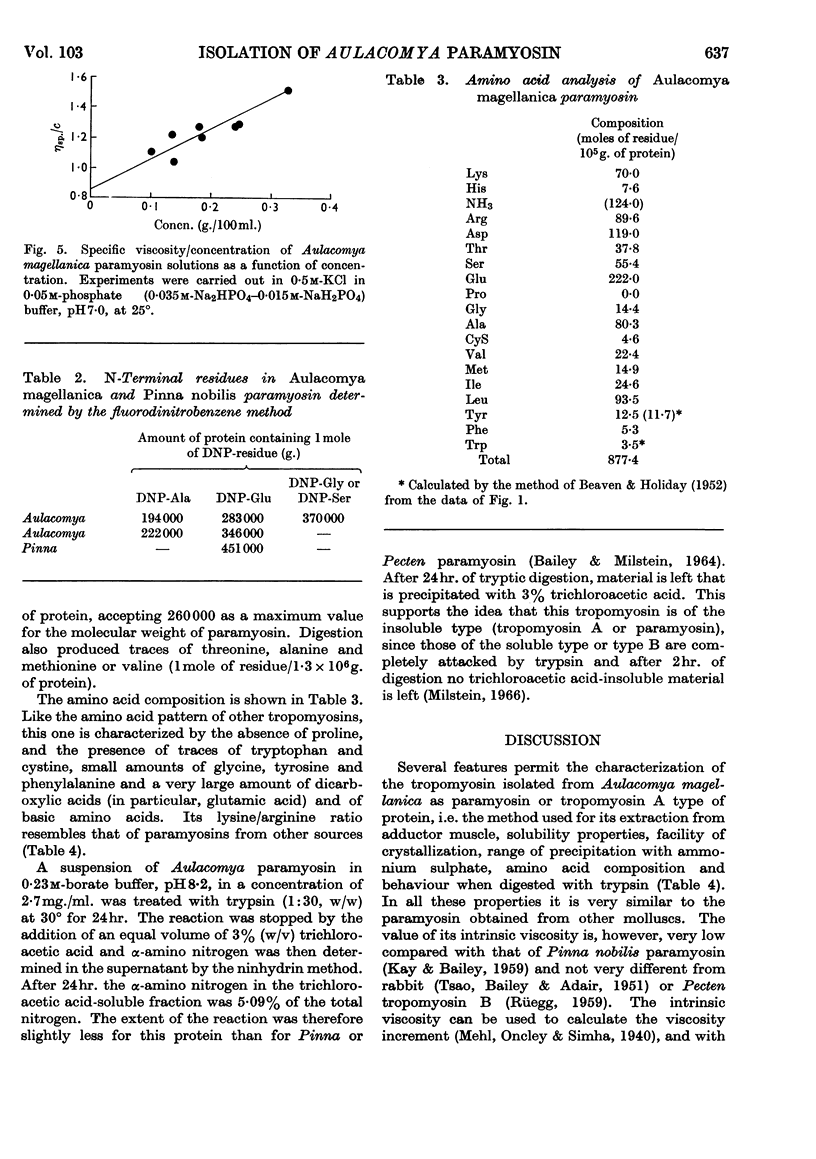
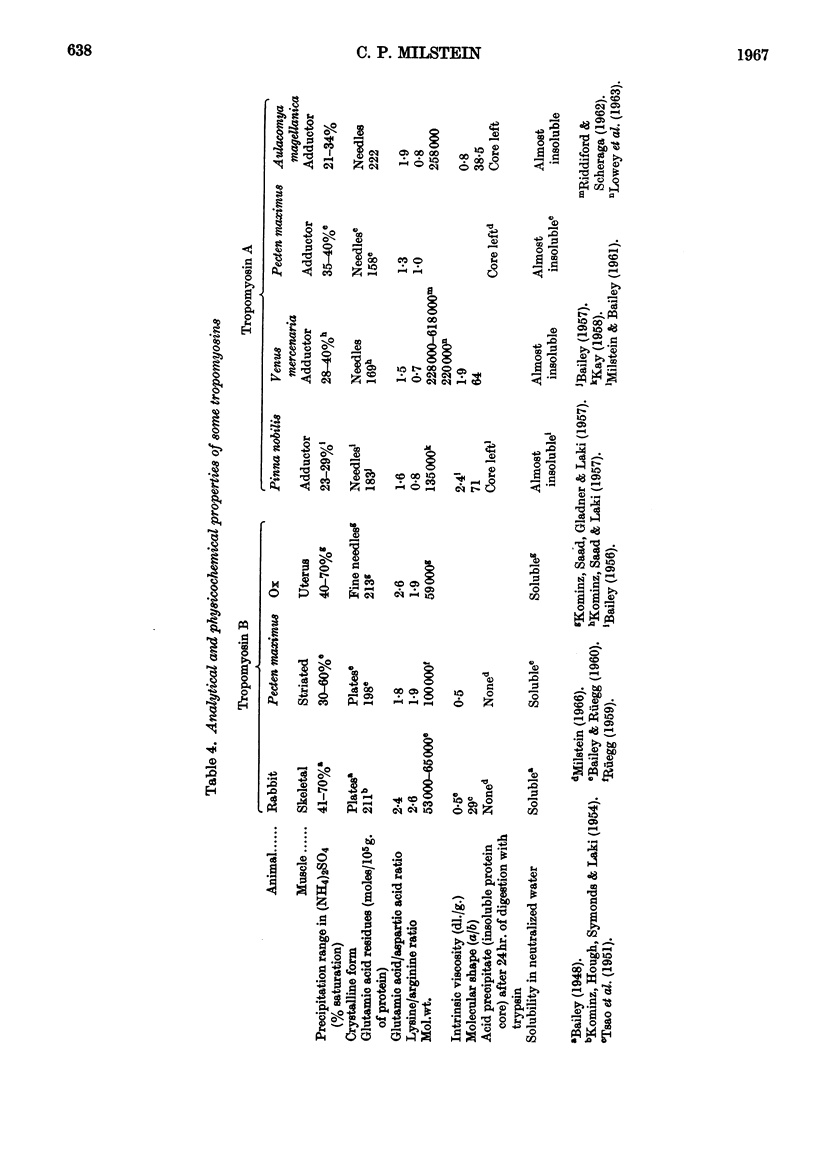
Tropomyosin A or paramyosin has been isolated from the adductor muscle of Aulacomya magellanica. It has in common with other tropomyosins A the method used for extracting it from adductor muscle, its solubility, facility of crystallization, ammonium sulphate range of precipitation, amino acid composition and behaviour when digested with trypsin. As a particular feature it exhibits an unusual low viscosity for this type of tropomyosin. Its molecular weight, determined by the Archibald approach-to-sedimentation-equilibrium method, is 258000±16000.
Full text
PDF


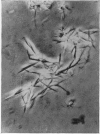
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAILEY K., DEMILSTEIN C. P., KAY C. M., SMILLIE L. B. CHARACTERIZATION OF A TRYPTIC FRAGMENT ISOLATED FROM THE INSOLUBLE TROPOMYOSIN OF PINNA NOBILIS. Biochim Biophys Acta. 1964 Sep 4;90:503–520. doi: 10.1016/0304-4165(64)90230-2. [DOI] [PubMed] [Google Scholar]
- BAILEY K., DEMILSTEIN C. P. TRYPTIC HYDROLYSIS OF PARAMYOSIN FROM INVERTEBRATES: RATE AND EXTENT OF PROTEOLYSIS. Biochim Biophys Acta. 1964 Sep 4;90:492–502. doi: 10.1016/0304-4165(64)90229-6. [DOI] [PubMed] [Google Scholar]
- BAILEY K. End-group assay in some proteins of the keratin-myosin group. Biochem J. 1951 Jun;49(1):23–27. doi: 10.1042/bj0490023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAILEY K. Invertebrate tropomyosin. Biochim Biophys Acta. 1957 Jun;24(3):612–619. doi: 10.1016/0006-3002(57)90255-x. [DOI] [PubMed] [Google Scholar]
- BAILEY K., RUEGG J. C. Further chemical studies on the tropomyosins of lamellibranch muscle with special reference to Pecten maximus. Biochim Biophys Acta. 1960 Feb 26;38:239–245. doi: 10.1016/0006-3002(60)91237-3. [DOI] [PubMed] [Google Scholar]
- BEAR R. S., SELBY C. C. The structure of paramyosin fibrils according to x-ray diffraction. J Biophys Biochem Cytol. 1956 Jan 25;2(1):55–69. doi: 10.1083/jcb.2.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLACKBURN S., LOWTHER A. G. The separation of N-2:4-dinitrophenly amino-acids on paper chromatograms. Biochem J. 1951 Jan;48(1):126–128. doi: 10.1042/bj0480126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey K. Tropomyosin: a new asymmetric protein component of the muscle fibril. Biochem J. 1948;43(2):271–279. doi: 10.1042/bj0430271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHIBNALL A. C., MANGAN J. L., REES M. W. Studies on the amide and C-terminal residues in proteins. 2. The ammonia nitrogen and amide nitrogen of various native protein preparations. Biochem J. 1958 Jan;68(1):111–114. doi: 10.1042/bj0680111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN C., HOLMES K. C. X-ray diffraction evidence for alpha-helical coiled-coils in native muscle. J Mol Biol. 1963 May;6:423–432. doi: 10.1016/s0022-2836(63)80053-4. [DOI] [PubMed] [Google Scholar]
- Chibnall A. C., Rees M. W., Williams E. F. The total nitrogen content of egg albumin and other proteins. Biochem J. 1943 Sep;37(3):354–359. doi: 10.1042/bj0370354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE MILSTEIN C. P., BAILEY K. Isolation and characterisation of a tryptic core from the insoluble tropomyosin of Pinna nobilis. Biochim Biophys Acta. 1961 May 13;49:412–413. doi: 10.1016/0006-3002(61)90150-0. [DOI] [PubMed] [Google Scholar]
- De Milstein C. P. Tryptic digestion of tropomyosin A, tropomyosin B and myosin. Nature. 1966 Feb 5;209(5023):614–615. doi: 10.1038/209614a0. [DOI] [PubMed] [Google Scholar]
- JOHNSON W. H., KAHN J. S., SZENTGYORGYI A. G. Paramyosin and contraction of catch muscles. Science. 1959 Jul 17;130(3368):160–161. doi: 10.1126/science.130.3368.160. [DOI] [PubMed] [Google Scholar]
- KAWERAU E., WIELAND T. Conservation of amino-acid chromatograms. Nature. 1951 Jul 14;168(4263):77–78. doi: 10.1038/168077b0. [DOI] [PubMed] [Google Scholar]
- KAY C. M., BAILEY K. Further physicochemical studies on Pinna nobilis tropomyosin. Biochim Biophys Acta. 1959 Jan;31(1):20–25. doi: 10.1016/0006-3002(59)90434-2. [DOI] [PubMed] [Google Scholar]
- KAY C. M. The partial specific volume of muscle proteins. Biochim Biophys Acta. 1960 Mar 11;38:420–427. doi: 10.1016/0006-3002(60)91277-4. [DOI] [PubMed] [Google Scholar]
- KOMINZ D. R., HOUGH A., SYMONDS P., LAKI K. The amino acid composition of action, myosin, tropomyosin and the meromyosins. Arch Biochem Biophys. 1954 May;50(1):148–159. doi: 10.1016/0003-9861(54)90017-x. [DOI] [PubMed] [Google Scholar]
- KOMINZ D. R., SAAD F., GLADNER J. A., LAKI K. Mammalian tropomyosins. Arch Biochem Biophys. 1957 Jul;70(1):16–28. doi: 10.1016/0003-9861(57)90075-9. [DOI] [PubMed] [Google Scholar]
- LOCKER R. H. C-Terminal groups in myosin, tropomyosin and actin. Biochim Biophys Acta. 1954 Aug;14(4):533–542. doi: 10.1016/0006-3002(54)90233-4. [DOI] [PubMed] [Google Scholar]
- LOWEY S., KUCERA J., HOLTZER A. ON THE STRUCTURE OF THE PARAMYOSIN MOLECULE. J Mol Biol. 1963 Sep;7:234–244. doi: 10.1016/s0022-2836(63)80003-0. [DOI] [PubMed] [Google Scholar]
- Mehl J. W., Oncley J. L., Simha R. VISCOSITY AND THE SHAPE OF PROTEIN MOLECULES. Science. 1940 Aug 9;92(2380):132–133. doi: 10.1126/science.92.2380.132. [DOI] [PubMed] [Google Scholar]
- PAULING L., COREY R. B. Stable configurations of polypeptide chains. Proc R Soc Lond B Biol Sci. 1953 Mar 11;141(902):21–33. doi: 10.1098/rspb.1953.0012. [DOI] [PubMed] [Google Scholar]
- PORTER R. R. A chemical study of rabbit antiovalbumin. Biochem J. 1950 Apr;46(4):473–478. doi: 10.1042/bj0460473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R. R., Sanger F. The free amino groups of haemoglobins. Biochem J. 1948;42(2):287–294. doi: 10.1042/bj0420287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F. The free amino groups of insulin. Biochem J. 1945;39(5):507–515. doi: 10.1042/bj0390507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSAO T. C., BAILEY K., ADAIR G. S. The size, shape and aggregation of tropomyosin particles. Biochem J. 1951 Jun;49(1):27–36. doi: 10.1042/bj0490027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods E. F. The dissociation of tropomyosin by urea. J Mol Biol. 1966 Apr;16(2):581–584. doi: 10.1016/s0022-2836(66)80199-7. [DOI] [PubMed] [Google Scholar]