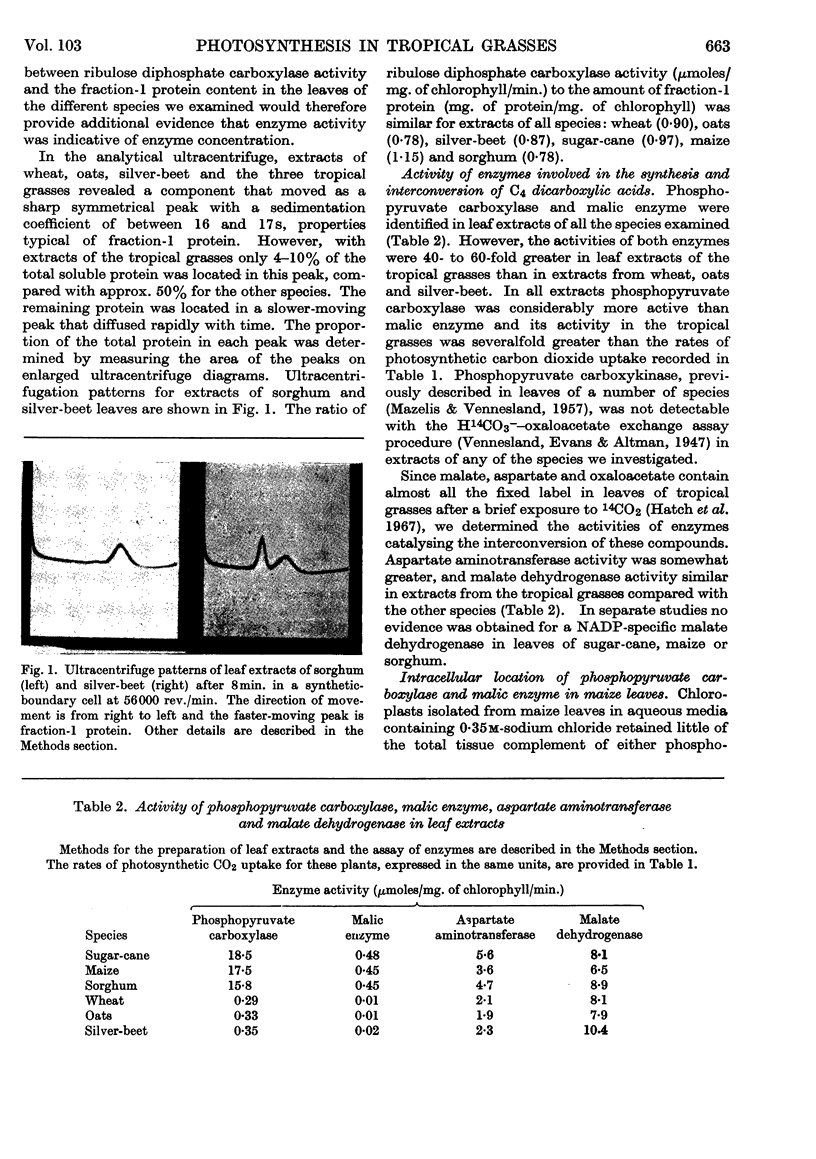
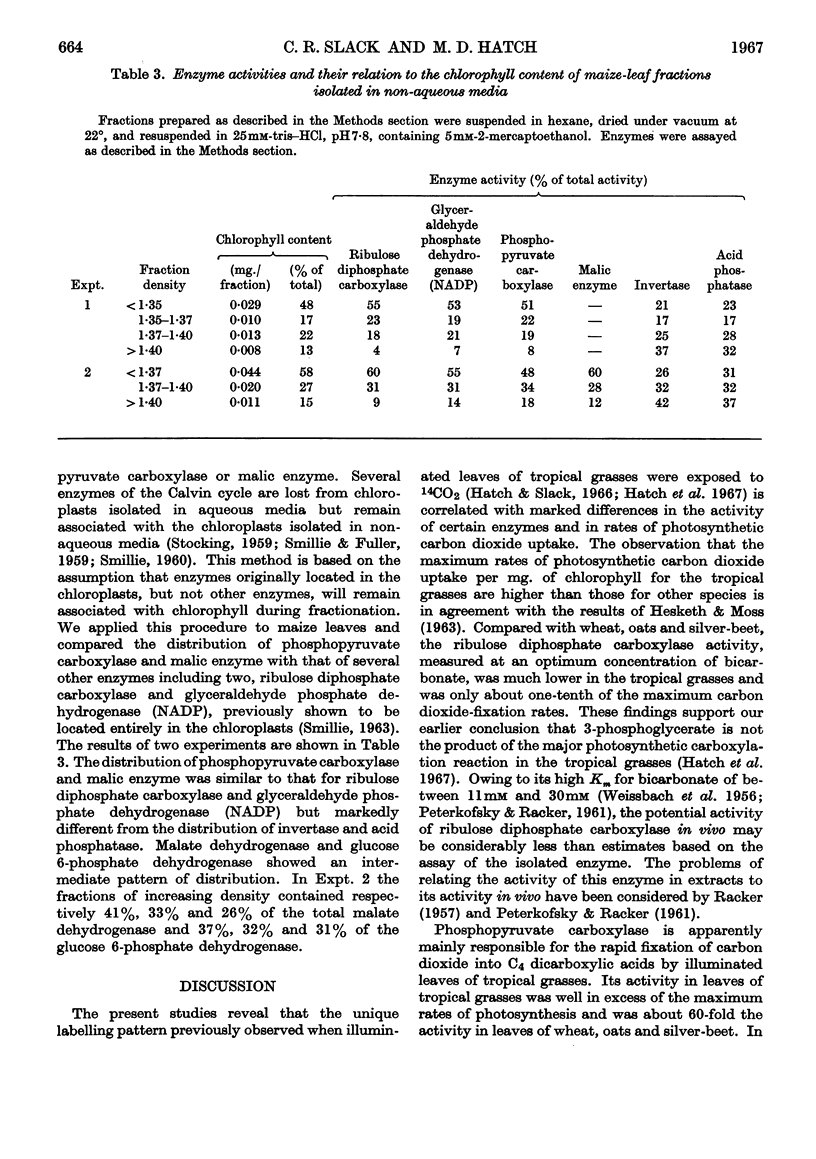
Abstract
1. The activity per unit of chlorophyll of certain carboxylases, and of other enzymes involved in photosynthesis, was determined in leaf extracts of the tropical grasses, sugar-cane, maize and sorghum, and compared with the activities for wheat, oat and silver-beet. Maximum rates of photosynthetic carbon dioxide uptake were also measured for comparison with enzyme activities. 2. Phosphopyruvate carboxylase activity was about 60 times greater in the tropical grasses than in wheat, oat and silver-beet and was severalfold higher than the rates of photosynthetic carbon dioxide uptake. Most of the enzyme was located in the chloroplast fraction of cell extracts. 3. Phosphopyruvate carboxylase was apparently the major photosynthetic carbon dioxide-fixing enzyme in the tropical grasses, although malic enzyme may contribute to a lesser extent. 4. Tropical grasses contained less than one-tenth of the ribulose diphosphate carboxylase activity present in wheat, oat and silver-beet. For the tropical grasses this activity, determined with a saturating concentration of bicarbonate, was approx. 10% of the rate of photosynthesis. 5. The fraction-1 protein content of leaf extracts paralleled the ribulose diphosphate carboxylase activity. 6. In contrast, the activity of several other enzymes of the Calvin cycle was similar in the different species examined.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R. A., Kornberg H. L. Net formation of phosphoenolpyruvate from pyruvate by Escherichia coli. Biochim Biophys Acta. 1965 Jul 8;104(2):618–620. doi: 10.1016/0304-4165(65)90374-0. [DOI] [PubMed] [Google Scholar]
- HURWITZ J., WEISSBACH A., HORECKER B. L., SMYRNIOTIS P. Z. Spinach phosphoribulokinase. J Biol Chem. 1956 Feb;218(2):769–783. [PubMed] [Google Scholar]
- Hatch M. D., Sacher J. A., Glasziou K. T. Sugar Accumulation Cycle in Sugar Cane. I. Studies on Enzymes of the Cycle. Plant Physiol. 1963 May;38(3):338–343. doi: 10.1104/pp.38.3.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch M. D., Slack C. R., Johnson H. S. Further studies on a new pathway of photosynthetic carbon dioxide fixation in sugar-cane and its occurrence in other plant species. Biochem J. 1967 Feb;102(2):417–422. doi: 10.1042/bj1020417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch M. D., Slack C. R. Photosynthesis by sugar-cane leaves. A new carboxylation reaction and the pathway of sugar formation. Biochem J. 1966 Oct;101(1):103–111. doi: 10.1042/bj1010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortschak H. P., Hartt C. E., Burr G. O. Carbon Dioxide Fixation in Sugarcane Leaves. Plant Physiol. 1965 Mar;40(2):209–213. doi: 10.1104/pp.40.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LYTTLETON J. W., TS'O P. O. The localization of fraction I protein of green leaves in the chloroplasts. Arch Biochem Biophys. 1958 Jan;73(1):120–126. doi: 10.1016/0003-9861(58)90246-7. [DOI] [PubMed] [Google Scholar]
- Mazelis M., Vennesland B. Carbon Dioxide Fixation into Oxalacetate in Higher Plants. Plant Physiol. 1957 Nov;32(6):591–600. doi: 10.1104/pp.32.6.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OGSTON A. G., PHELPS C. F. Exclusion of inulin from solutions of hyaluronic acid. Nature. 1960 Sep 17;187:1024–1024. doi: 10.1038/1871024a0. [DOI] [PubMed] [Google Scholar]
- Peterkofsky A., Racker E. The reductive pentose phosphate cycle. III. Enzyme activities in cell-free extracts of photosynthetic organisms. Plant Physiol. 1961 Jul;36(4):409–414. doi: 10.1104/pp.36.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RACKER E., SCHROEDER E. A. The reductive pentose phosphate cycle. II. Specific C-1 phosphatases for fructose 1,6-diphosphate and sedoheptulose 1,7-diphosphate. Arch Biochem Biophys. 1958 Apr;74(2):326–344. doi: 10.1016/0003-9861(58)90004-3. [DOI] [PubMed] [Google Scholar]
- RACKER E. The reductive pentose phosphate cycle. I. Phosphoribulokinase and ribulose diphosphate carboxylase. Arch Biochem Biophys. 1957 Jul;69:300–310. doi: 10.1016/0003-9861(57)90496-4. [DOI] [PubMed] [Google Scholar]
- SINGER S. J., EGGMAN L., CAMPBELL J. M., WILDMAN S. G. The proteins of green leaves. IV. A high molecular weight protein comprising a large part of the cytoplasmic proteins. J Biol Chem. 1952 May;197(1):233–239. [PubMed] [Google Scholar]
- Smillie R. M., Fuller R. C. Ribulose 1,5-Diphosphate Carboxylase Activity in Relation to Photosynthesis by Intact Leaves And Isolated Chloroplasts. Plant Physiol. 1959 Nov;34(6):651–656. doi: 10.1104/pp.34.6.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocking C. R. Chloroplast Isolation in Nonaqueous Media. Plant Physiol. 1959 Jan;34(1):56–61. doi: 10.1104/pp.34.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISSBACH A., HORECKER B. L., HURWITZ J. The enzymatic formation of phosphoglyceric acid from ribulose diphosphate and carbon dioxide. J Biol Chem. 1956 Feb;218(2):795–810. [PubMed] [Google Scholar]
- WOLFE R. G., NEILANDS J. B. Some molecular and kinetic properties of heart malic dehydrogenase. J Biol Chem. 1956 Jul;221(1):61–69. [PubMed] [Google Scholar]