Abstract
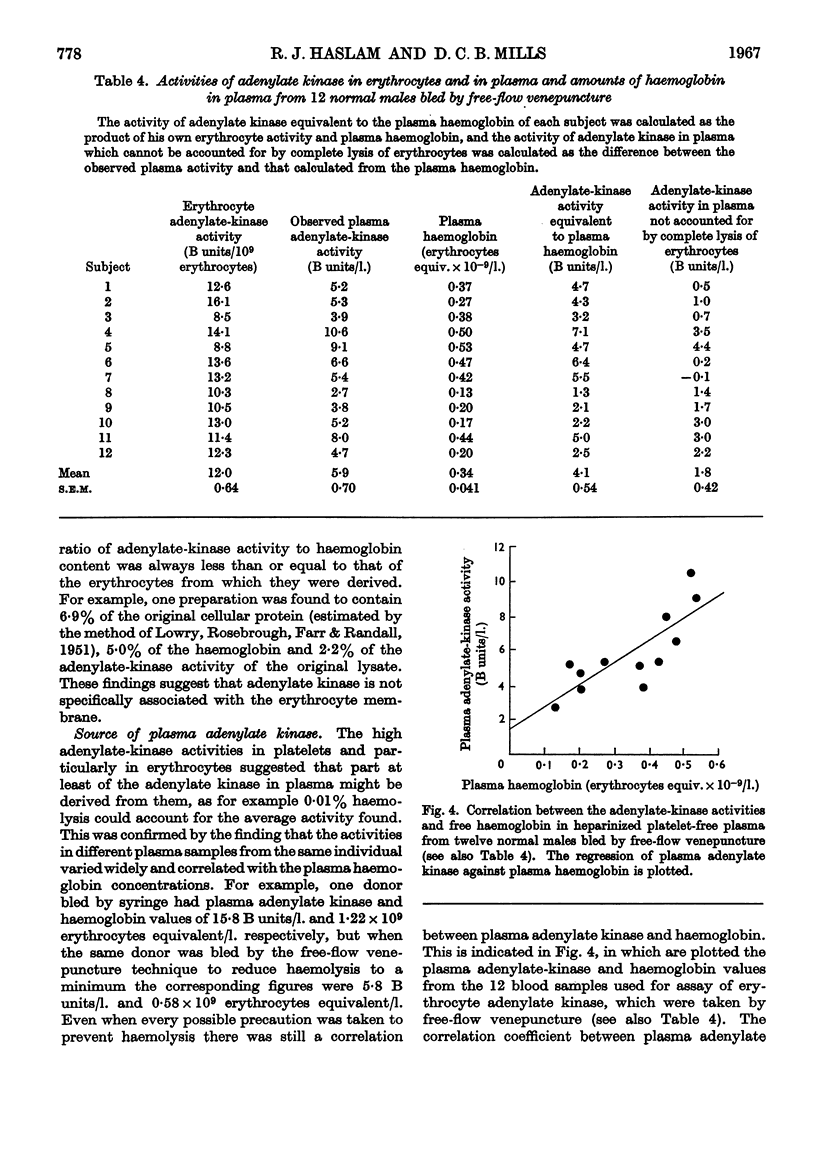
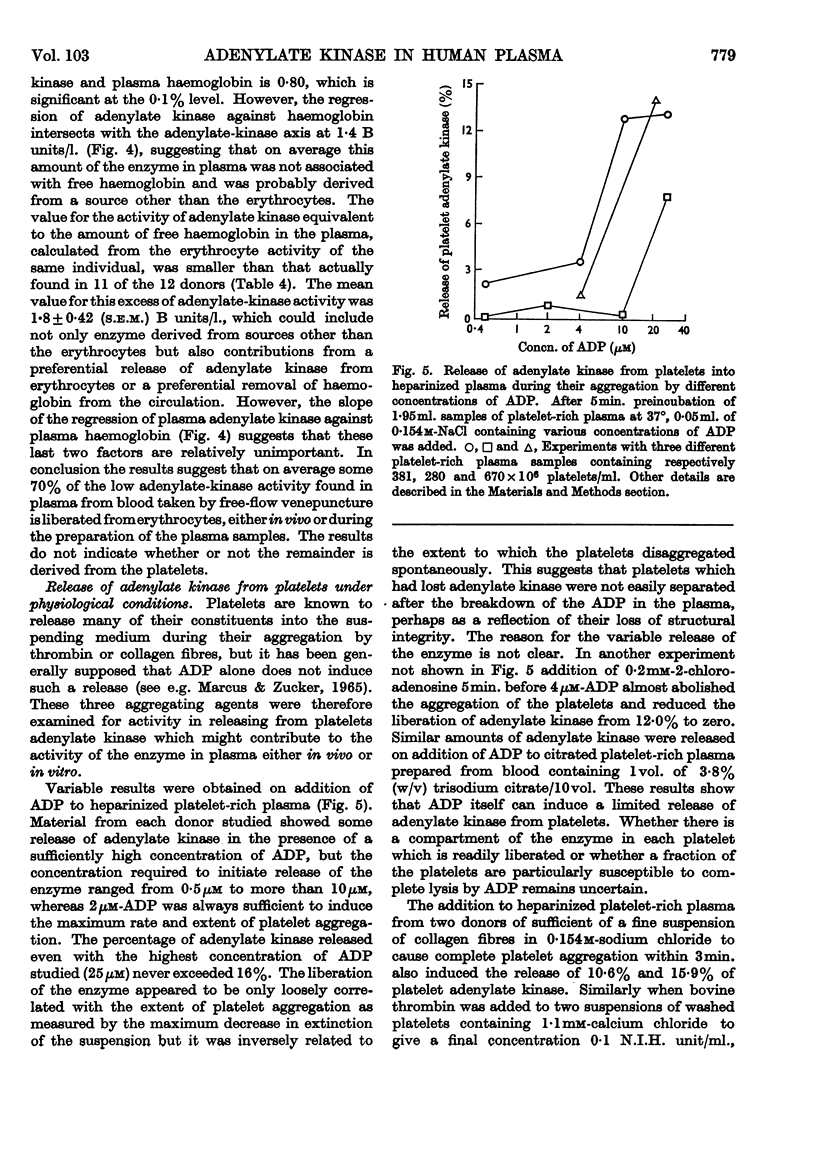
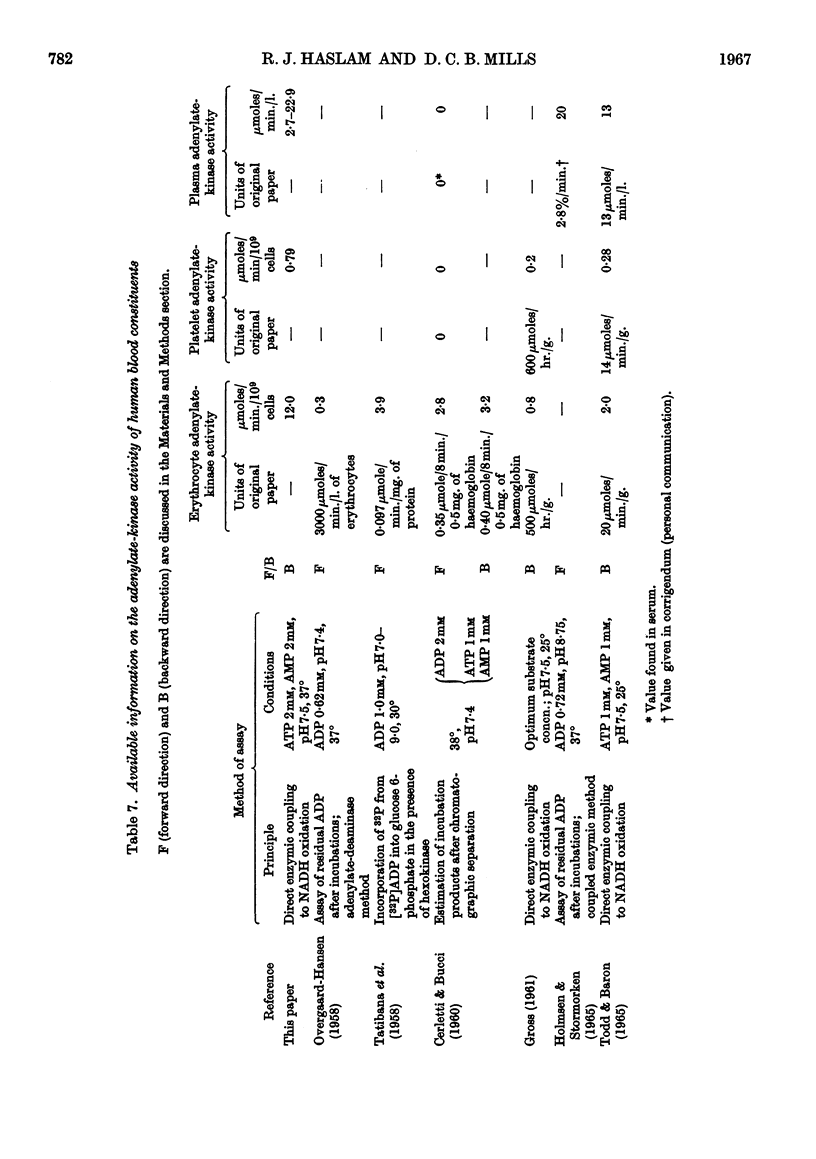
1. Adenylate kinase (EC 2.7.4.3) has been shown to be present in human plasma obtained by conventional means and the adenylate-kinase activities of plasma and of lysed and intact human platelets and erythrocytes have been measured at 37° by sensitive spectrophotometric methods. 2. The activities found in plasma ranged from 2·7 to 22·9μmoles of ADP formed/min./l. and in lysed platelets and lysed erythrocytes mean values of 0·79 and 12·0μmoles of ADP formed/min./109 cells respectively were found. Intact platelets and erythrocytes showed little or no activity. 3. The apparent Km of plasma adenylate kinase for ADP was found to be 1·4–1·6mm. 4. The adenylate-kinase activity of plasma was correlated with the free haemoglobin present and the larger part of the activity could be accounted for by haemolysis occurring either during the withdrawal of the blood or in vivo. 5. Aggregation of platelets by ADP, collagen fibres or thrombin released up to 16% of the platelet adenylate kinase into the suspending medium. 6. Measurement of the rate of breakdown of 1·6μm-ADP in plasma gave values of about 0·1mμ-mole/min./ml. This was not increased by addition of sufficient erythrocyte lysate to increase the activity of plasma adenylate kinase five to ten times. 7. It was concluded that the activity of adenylate kinase found in plasma, even after aggregation of the platelets, is insufficient to account for the rate of breakdown of low concentrations of ADP usually observed, and that another enzyme is responsible for this process.
Full text
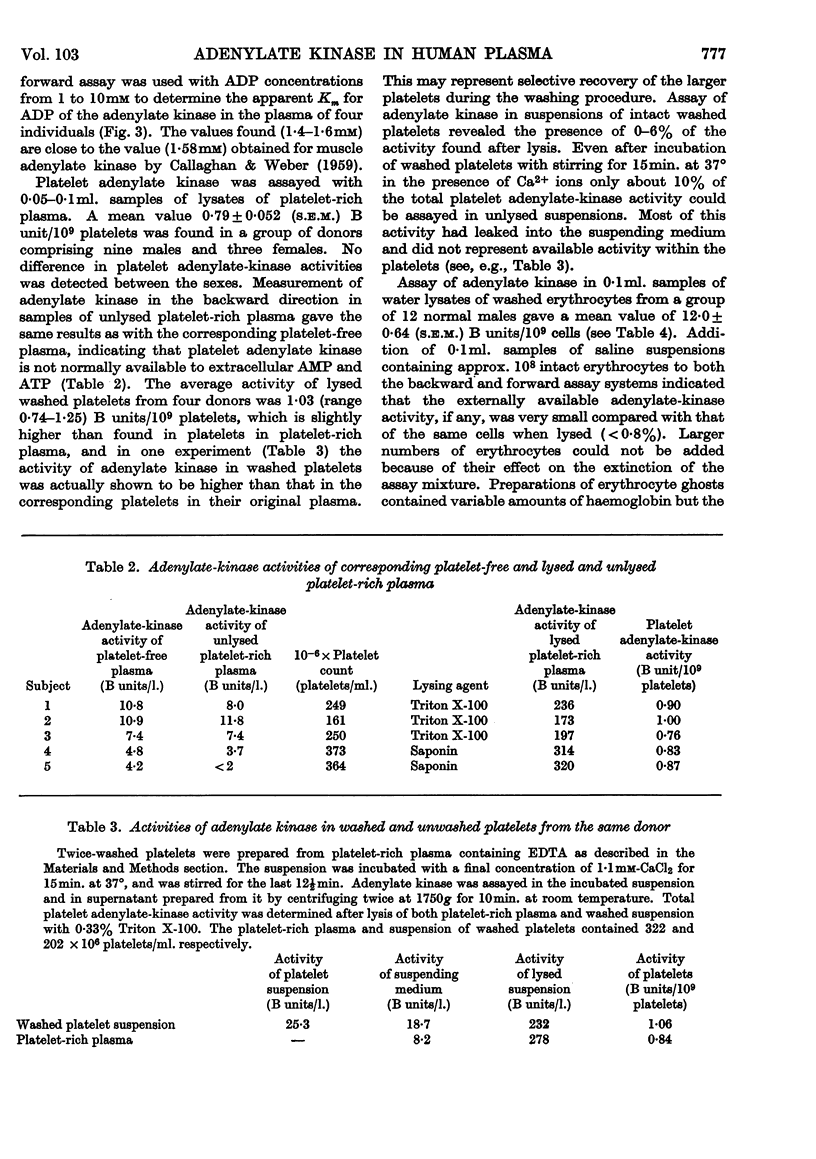
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASKARI A., FRATANTONI J. C. EFFECT OF NA+ AND K+ ON THE ADENYLATE KINASE OF HUMAN ERYTHROCYTES. Proc Soc Exp Biol Med. 1964 Jul;116:751–753. [PubMed] [Google Scholar]
- Allen R. J. The estimation of phosphorus. Biochem J. 1940 Jun;34(6):858–865. doi: 10.1042/bj0340858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORN G. V. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature. 1962 Jun 9;194:927–929. doi: 10.1038/194927b0. [DOI] [PubMed] [Google Scholar]
- CALLAGHAN O. H., WEBER G. Kinetic studies on rabbitmuscle myokinase. Biochem J. 1959 Nov;73:473–485. doi: 10.1042/bj0730473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CERLETTI P., BUCCI E. Adenylate kinase of mammalian erythrocytes. Biochim Biophys Acta. 1960 Feb 12;38:45–50. doi: 10.1016/0006-3002(60)91194-x. [DOI] [PubMed] [Google Scholar]
- CERLETTI P., DE RITIS G. Enzymes of the red blood cell membrane: adenylate kinase activity. Clin Chim Acta. 1962 May;7:402–405. doi: 10.1016/0009-8981(62)90041-4. [DOI] [PubMed] [Google Scholar]
- Green D. E., Murer E., Hultin H. O., Richardson S. H., Salmon B., Brierley G. P., Baum H. Association of integrated metabolic pathways with membranes. I. Glycolytic enzymes of the red blood corpuscle and yeast. Arch Biochem Biophys. 1965 Dec;112(3):635–647. doi: 10.1016/0003-9861(65)90107-4. [DOI] [PubMed] [Google Scholar]
- HANKS G. E., CASSELL M., RAY R. N., CHAPLIN H., Jr Further modification of the benzidine method for measurement of hemoglobin in plasma; definitionn of a new range of normal values. J Lab Clin Med. 1960 Sep;56:486–498. [PubMed] [Google Scholar]
- HASLAM R. J. ROLE OF ADENOSINE DIPHOSPHATE IN THE AGGREGATION OF HUMAN BLOOD-PLATELETS BY THROMBIN AND BY FATTY ACIDS. Nature. 1964 May 23;202:765–768. doi: 10.1038/202765a0. [DOI] [PubMed] [Google Scholar]
- Holmsen H., Stormorken H., Goote T. M. Kinetic studies on the breakdown of adenosine diphosphate in human plasma. Scand J Clin Lab Invest. 1965;17(Suppl):138+–138+. [PubMed] [Google Scholar]
- Ireland D. M., Mills D. C. Detection and determination of adenosine diphosphate and related substances in plasma. Biochem J. 1966 May;99(2):283–296. doi: 10.1042/bj0990283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KASHKET S., DENSTEDT O. F. The metabolism of the erythrocyte. XV. Adenylate kinase of the erythrocyte. Can J Biochem Physiol. 1958 Oct;36(10):1057–1064. [PubMed] [Google Scholar]
- KERBY G. P., TAYLOR S. M. THE ROLE OF HUMAN PLATELETS AND PLASMA IN THE METABOLISM OF ADENOSINE DIPHOSPHATE AND MONOPHOSPHATE ADDED IN VITRO. Thromb Diath Haemorrh. 1964 Dec 31;12:510–523. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- OLIVER I. T. A spectrophotometric method for the determination of creatine phosphokinase and myokinase. Biochem J. 1955 Sep;61(1):116–122. doi: 10.1042/bj0610116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEN A. K., POST R. L. STOICHIOMETRY AND LOCALIZATION OF ADENOSINE TRIPHOSPHATE-DEPENDENT SODIUM AND POTASSIUM TRANSPORT IN THE ERYTHROCYTE. J Biol Chem. 1964 Jan;239:345–352. [PubMed] [Google Scholar]
- Turtle J. R., Firkin B. G. Changes in nucleotides following the addition of adenosine diphosphate to platelet suspensions. Australas Ann Med. 1965 Nov;14(4):282–285. doi: 10.1111/imj.1965.14.4.282. [DOI] [PubMed] [Google Scholar]



