Abstract
We susceptibility tested 17 clinical isolates of Aspergillus fumigatus, for most of which MICs of itraconazole were elevated (MIC at which 50% of the isolates tested are inhibited, 16 μg/ml), against itraconazole, posaconazole, ravuconazole, and voriconazole. Posaconazole was the most active against itraconazole-susceptible isolates. A complex pattern of cross-resistance and hypersusceptibility was seen with voriconazole and ravuconazole, suggesting marked differences in activity and mechanisms of resistance.
Invasive aspergillosis is now the most common invasive mould infection worldwide and is increasing rapidly in frequency (4). Amphotericin B and itraconazole (ITC) were the only two agents licensed for the treatment of Aspergillus infections, until the recent licensure of caspofungin for salvage usage. Response rates are poor (typically 35 to 55%). Resistance has been described and may contribute to failure (9). Several promising new agents are undergoing clinical trials, including three new azoles with good activity against Aspergillus in vitro and in animal models: posaconazole (SCH 56592; PCZ), voriconazole (UK 109496; VRC), and ravuconazole (BMS-207147; RVZ). In vivo resistance to ITC (2, 6) and elevated VRC MICs (12) have already been described for Aspergillus fumigatus clinical isolates, as well as elevated ITC MICs for Aspergillus nidulans (3). Our own work over several years has shown a maximum ITC resistance rate of 4.2% in Aspergillus spp. (MIC > 4 μg/ml), using an inoculum that may overestimate resistance. Of 900 isolates of A. fumigatus tested in the recent literature against ITC, 2.1% are reported to be resistant (9). No study has investigated azole cross-resistance in more than a handful of clinical Aspergillus isolates.
We have tested 17 different clinical isolates of A. fumigatus, for which MICs range from 0.13 to 16 μg/ml (ITC, VRC, RVZ, and PCZ). Eleven of those isolates we have defined as resistant to ITC in vitro (MIC > 4 μg/ml). Breakpoints have yet to be validated in vivo. We have employed a final inoculum of 5 × 104 CFU/ml, 10-fold lower than in previous work with A. fumigatus, in which we used 5 × 105 CFU/ml, for three reasons. First, a wider range of MICs is obtained as the lower inoculum lowers MICs and as there is an upper limit of ITC solubility (8 μg/ml). Second, unpublished and published work (10) from our lab shows that, in some but not all Aspergillus flavus isolates, a final inoculum of 5 × 105 CFU/ml disproportionately increases MICs (due to trailing), falsely suggesting resistance. Third, a recent NCCLS reproducibility study showed that, with the final inoculum range recommended by the NCCLS, between 0.4 and 5 × 104 CFU/ml, good reproducibility is obtained and resistance is identified (7). Fourth, the lowest NCCLS inoculum may fail to identify elevated MICs for some isolates because the MIC distribution is too narrow.
All strains were obtained from different patients, with the exception of FA/5211, FA/6919, and FA/7075 from one patient and 1112 and 1237 from another, in both of whom resistant strains appeared on therapy with ITC (1). ITC (Janssen Research Foundation, Beerse, Belgium), VRC (Pfizer, Sandwich, United Kingdom), PCZ (Schering-Plough Research Institute, Bloomfield, N.J.), and RVZ (Bristol-Myers Squibb Company, Princeton, N.J.) were obtained as standard powders from their respective manufacturers. They were dissolved in dimethyl sulfoxide (Sigma, Poole, United Kingdom). In vitro susceptibility testing was performed with a broth microdilution-based method, validated in vivo in our laboratory, that was capable of detecting ITC resistance in A. fumigatus (5). RPMI 1640 plus 2% glucose (pH 7.0) as testing medium, a final inoculum of 5 × 104 CFU/ml, a 48-h incubation period at 37°C, and a visual no-growth endpoint were used. Dilution ranges were 8 to 0.008 μg/ml for ITC, 4 to 0.004 μg/ml for PCZ, 64 to 0.06 μg/ml for VRC, and 32 to 0.03 μg/ml for RVZ. Reproducibility studies showed that, for all four drugs, 100% (eight out of eight) of isolates retested gave a result within one twofold dilution.
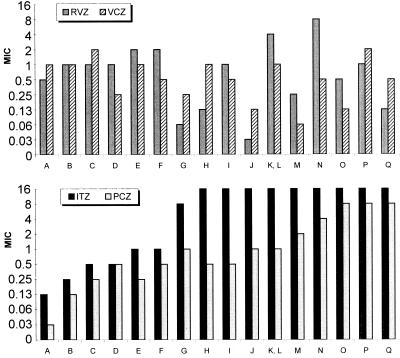
Median MICs (in micrograms per milliliter) were ITC, 16; PCZ, 1; VRC, 0.5; and RVZ, 1. Elevated ITC MICs were uniformly associated with elevations in PCZ MICs of 4- to 256-fold, as the PCZ MICs for isolates fully susceptible to ITC generally are 0.03 μg/ml (Fig. 1).The impact of this MIC shift has been documented in vivo (11). However, the clinical implications of elevated PCZ MICs are not known, since achievable serum drug concentrations far exceed this concentration. High doses of PCZ could overcome elevated MICs, as shown in a murine model for the ITC-resistant isolate AF90 (PCZ MIC, 1 μg/ml) (11). A recent study conducted on azole cross-resistance, employing a significant number of laboratory-selected isolates for which ITC MICs were elevated, showed only a slight (two- to threefold) increase in PCZ MICs (8).
FIG. 1.
MICs for the A. fumigatus isolates of ITC (ITZ), PCZ, VRC (VCZ), and RVZ are given in micrograms per milliliter. A, AF41 (fully susceptible); B, F/6631; C, F/5211; D, IHEM 17905; E, SO/3626; F, F/7763; G, Br181; H, SO/3827; I, SO/3829; J, Br130; K, Br128; L, AF90; M, AF72; N, AF1422; O, IHEM 17907; P, F/6919; and Q, F/7075.
Elevated ITC MICs were not usually associated with elevated MICs of VRC or RVZ. For only 3 out of the 11 ITC in vitro resistant isolates did RVZ MICs increase by more than twofold (MIC, 4 to 8 μg/ml), compared to the result for the most ITC-susceptible isolate (MIC, 0.5 μg/ml). VRC MICs varied between 0.06 and 2 μg/ml, and RVZ MICs ranged from 0.03 to 8 μg/ml. The susceptibility pattern of the isolates against VRC and RVZ was very similar, suggesting similar modes of action and mechanisms of resistance. Interestingly, the lowest VRC and RVZ MICs were seen for highly ITC-resistant isolates. For 5 of 11 and 7 of 11 of the ITC-resistant isolates, RVZ and VRC MICs (2- to 16-fold), respectively, decreased, compared to the MICs for the most ITC-susceptible isolate. For the isolate for which the RCZ MIC was highest, the VRC MIC was low, but the ITC and PCZ MICs were both elevated.
These data indicate substantial heterogeneity in susceptibility among isolates of A. fumigatus and suggest the existence of different mechanisms of resistance against antifungal azoles. As might be expected from structural considerations, the susceptibility patterns of ITC and PCZ are similar (although PCZ is consistently more active than ITC), as are those of VRC and RVZ. However, individual variations of MICs are not entirely predictable and testing of each isolate to each drug is likely to be valuable. Optimizing therapy for those few patients in whom an isolate is grown, by selecting the most active azole drug, is now feasible. Reproducibility of results and definition of breakpoints will be important for clinical acceptance. Clearly, subtle variations in the specific mode of action of each azole against A. fumigatus isolate are revealed by this work, but they are barely understood. New studies are warranted in order to analyze the mechanisms involved in these complex azole susceptibility patterns.
This work has been supported by the European Commission Training and Mobility of Researchers grant FMRX-CT970145 Eurofung and the Fungal Research Trust.
REFERENCES
- 1.Dannaoui, E., E. Borel, M. Monier, M. Piens, S. Picot, and F. Persat. 2001. Acquired itraconazole resistance in Aspergillus fumigatus. J. Antimicrob. Chemother. 47:333-340. [DOI] [PubMed] [Google Scholar]
- 2.Dannaoui, E., E. Borel, F. Persat, M. F. Monier, and M. A. Piens. 1999. In-vivo itraconazole resistance of Aspergillus fumigatus in systemic murine aspergillosis. EBGA Network. European research group on Biotypes and Genotypes of Aspergillus fumigatus. J. Med. Microbiol. 48:1087-1093. [DOI] [PubMed] [Google Scholar]
- 3.Dannaoui, E., F. Persat, M. F. Monier, E. Borel, M. A. Piens, and S. Picot. 1999. In-vitro susceptibility of Aspergillus spp. isolates to amphotericin B and itraconazole. J. Antimicrob. Chemother. 44:553-555. [DOI] [PubMed] [Google Scholar]
- 4.Denning, D. W. 1998. Invasive aspergillosis. Clin. Infect. Dis. 26:781-805. [DOI] [PubMed] [Google Scholar]
- 5.Denning, D. W., S. A. Radford, K. L. Oakley, L. Hall, E. M. Johnson, and D. W. Warnock. 1997. Correlation between in-vitro susceptibility testing to itraconazole and in-vivo outcome of Aspergillus fumigatus infection. J. Antimicrob. Chemother. 40:401-414. [DOI] [PubMed] [Google Scholar]
- 6.Denning, D. W., K. Venkateswarlu, K. L. Oakley, M. J. Anderson, N. J. Manning, D. A. Stevens, D. W. Warnock, and S. L. Kelly. 1997. Itraconazole resistance in Aspergillus fumigatus. Antimicrob. Agents Chemother. 41:1364-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Espinel-Ingroff, A., M. Bartlett, V. Chaturvedi, M. Ghannoum, K. C. Hazen, M. A. Pfaller, M. Rinaldi, and T. J. Walsh. 2001. Optimal susceptibility testing conditions for detection of azole resistance in Aspergillus spp.: NCCLS collaborative evaluation. Antimicrob. Agents Chemother. 45:1828-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manavathu, E. K., J. L. Cutright, D. Loebenberg, and P. H. Chandrasekar. 2000. A comparative study of the in vitro susceptibilities of clinical and laboratory-selected resistant isolates of Aspergillus spp. to amphotericin B, itraconazole, voriconazole and posaconazole (SCH 56592). J. Antimicrob. Chemother. 46:229-234. [DOI] [PubMed] [Google Scholar]
- 9.Moore, C. B., N. Sayers, J. Mosquera, J. Slaven, and D. W. Denning. 2000. Antifungal drug resistance in Aspergillus. J. Infect. 41:203-220. [DOI] [PubMed] [Google Scholar]
- 10.Mosquera, J., P. A. Warn, J. Morrissey, C. B. Moore, C. Gil-Lamaignere, and D. W. Denning. 2001. Susceptibility testing of Aspergillus flavus: inoculum dependence with itraconazole and lack of correlation between susceptibility to amphotericin B in vitro and outcome in vivo. Antimicrob. Agents Chemother. 45:1456-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oakley, K. L., G. Morrissey, and D. W. Denning. 1997. Efficacy of SCH-56592 in a temporarily neutropenic murine model of invasive aspergillosis with an itraconazole-susceptible and an itraconazole-resistant isolate of Aspergillus fumigatus. Antimicrob. Agents Chemother. 41:1504-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verweij, P. E., M. Mensink, A. J. Rijs, J. P. Donnelly, J. F. Meis, and D. W. Denning. 1998. In-vitro activities of amphotericin B, itraconazole and voriconazole against 150 clinical and environmental Aspergillus fumigatus isolates. J. Antimicrob. Chemother. 42:389-392. [DOI] [PubMed] [Google Scholar]