Abstract
As a result of our search for new isoniazid derivatives with extended spectra of activity, we evaluated the in vitro antimycobacterial activities of isonicotinohydrazides (compounds 2) and their cyanoborane adducts (compounds 3), both obtained by the reaction of isonicotinoylhydrazones (compounds 1) with sodium cyanoborohydride. Most of the tested compounds displayed moderate to high activity against Mycobacterium tuberculosis H37Rv, with MICs ranging from 0.2 to 12.5 μg/ml. In particular, some hydrazides showed activity similar to that of rifampin (MIC = 0.2 μg/ml) and rather low cytotoxicity, so that they were generally shown to possess high safety indices. In contrast, the coordination to a cyanoborane (BH2CN) group (compounds 3) in general brought about a decrease in antimycobacterial activity, while cytotoxicity increased. Interestingly, selected compounds 1 to 3, mostly hydrazides (compounds 2), were effective in killing M. tuberculosis growing within macrophages at concentrations in culture medium which were much lower than the corresponding MICs. These compounds also displayed good activity against drug-resistant M. tuberculosis strains.
Tuberculosis (TB) is still a challenging worldwide health problem, and Mycobacterium tuberculosis remains one of the single most deadly human pathogens. The resurgence of TB over the last 15 years, even in industrialized countries where it was almost eradicated, has been favored by the pathogenic synergy with human immunodeficiency virus infection. In fact, TB and other atypical mycobacterioses are now diseases frequently associated with AIDS; human immunodeficiency virus infection significantly increases the risk that new or latent TB infections will progress to active diseases (5, 10–12).
The emergence of TB has also been accompanied by the appearance of single-drug-resistant (SDR) and multidrug-resistant strains of M. tuberculosis which are insensitive to one or more of the first-line anti-TB drugs (isoniazid [INH], rifampin, ethambutol, streptomycin, and pyrazinamide) (27). Indeed, a great amount of work has been done in order to acquire useful knowledge about the mechanisms of action of and resistance to available antitubercular agents (7, 13, 17–20, 22–24, 33). M. tuberculosis often becomes drug resistant as a consequence of spontaneous genetic mutations involving the molecular targets of drugs. The primary mechanism of multidrug resistance in TB is the accumulation of mutations in individual drug target genes (19). However, such knowledge is not sufficient to rationally overcome drug resistance in mycobacteria. In fact, currently, combinations of two or more anti-TB drugs are used to prevent the development of resistant mycobacteria; sometimes it is also necessary to resort to second-line drugs (ciprofloxacin, ethionamide, kanamycin, and aminosalicylic acid, etc.) (15, 25). Consequently, the present anti-TB regimen is rather complex and lengthy. In immunosuppressed patients, it is also unsatisfactory. All of these serious concerns require particular attention and stimulate the continuing search for new antimycobacterial agents and therapeutic regimens.
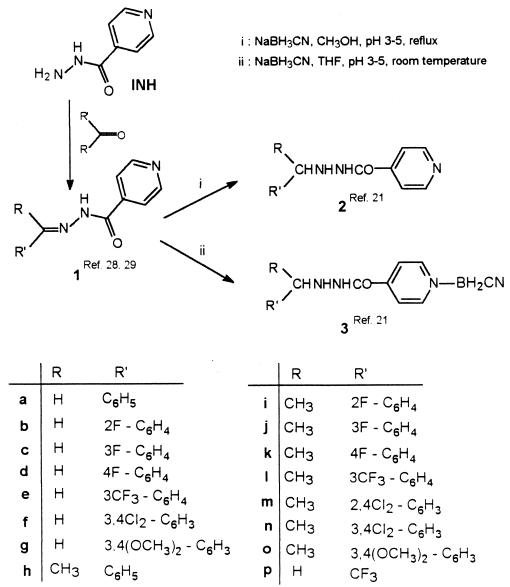
In the last few years, we have synthesized and pharmacologically explored numerous different lipophilic analogues of INH with the aim of finding new compounds with activity against TB as well as other AIDS-associated diseases (2, 3, 21, 28–31). In particular, we have recently reported on the synthesis of 2′-arylalkylisonicotinohydrazides (compounds 2) and their cyanoborane adducts (compounds 3) (Fig. 1) (21). We found that several of them have promising antiproliferative properties against several human tumor cell lines (31). We now report on the in vitro evaluation of their antimycobacterial activities. This was considered to be of interest since compounds 2 and 3, which maintain the isonicotinoyl moiety, a pharmacophore considered to be important in antitubercular activity, also have lipophilic moieties linked to 2′ N and pyridine N, which could potentially affect pharmacokinetics by facilitating the crossing of biomembranes or prolonging biological activities.
FIG. 1.
Structures of INH and analogues.
In the 1960s and 1970s, several 2′-monosubstituted isonicotinohydrazides were reported to possess appreciable in vivo antimycobacterial activity linked to the release of parental INH (8, 14, 16, 25). To our knowledge, cyanoboranes with such anti-infective properties have not been reported before.
MATERIALS AND METHODS
Chemical syntheses.
The synthesis of 2′-arylalkylisonicotinohydrazides (compounds 2) and 2′-arylalkylisonicotinohydrazide cyanoboranes (compounds 3) were performed according to previously reported procedures (21). Briefly, the treatment of isonicotinoylhydrazones (ISNEs) (compounds 1) with sodium cyanoborohydride (NaBH3CN) (molar ratio, 1:2) in anhydrous tetrahydrofuran (THF) at room temperature (reaction time, 30 min) produced 2′-arylalkylisonicotinohydrazide cyanoborane adducts (compounds 3) in high yields, whereas the same reaction carried out in refluxing methanol quantitatively provided 2′-arylalkylisonicotinohydrazides (compounds 2). Compounds 2 and 3 were characterized by means of infrared and 1H and 13C nuclear magnetic resonance (NMR) spectroscopies; data for the newly synthesized compounds are summarized in Table 1.
TABLE 1.
Physical-chemical data for newly synthesized compounds 2 and 3
| Compound | Formula | Yield (%) | mp (°C) | 1H NMR (δ, CDCl3); 13C NMR (δ, CDCl3)a |
|---|---|---|---|---|
| 2b | C13H12FN3O | 92 | 105–107 (THF-hexane) | 4.16 (s, 2H, CH2), 5.68 (br. s, 1H, 2′H), 7.04–7.41 (m, 4H, Ph), 7.55 (m, 2H, Hβ-Py), 8.18 (br. s, 1H, 1′H), 8.72 (m, 2H, Hα-Py); 49.4, 115.5 (JCF = 17.0), 120.9, 124.0, 124.2, 129.8, 131.3, 140.0, 150.2, 161.6 (JCF = 243.0), 165.3 (C O) |
| 2c | C13H12FN3O | 95 | 128–130 (THF-hexane) | 4.03 (s, 2H, CH2), 4.92 (br. s, 1H, 2′H), 6.91–7.24 (m, 4H, Ph), 7.55 (m, 2H, Hβ-Py), 8.56 (m, 2H, Hα-Py), 9.18 (br. s, 1H, 1′H); 55.0, 114.5, 115.5, 121.0, 124.4, 129.9, 139.8, 140.0, 150.1, 162.7 (JCF = 230.0), 165.4 (C O) |
| 2f | C13H11Cl2N3O | 88 | 158–160 (THF-hexane) | 4.08 (s, 2H, CH2), 4.82 (br. s, 1H, 2′H), 7.25 (dd J = 8.1 and 1.8, 1H, Ph), 7.42 (d J = 8.1, 1H, Ph), 7.52 (d J = 1.8, 1H, Ph), 7.58 (m, 2H, Hβ-Py), 8.75 (m, 2H, Hα-Py), 8.18 (br. s, 1H, 1′H); 54.5, 121.0, 128.2, 130.4, 130.7, 131.7, 132.5, 137.5, 139.9, 150.2, 165.5 (C O) |
| 2i | C14H14FN3O | 97 | Oil | 1.42 (d J = 6.6, 3H, CH3), 4.57 (q J = 6.6, 1H, CH), 4.89 (br. s, 1H, 2′H), 6.99–7.22 (m, 4H, Ph), 7.46 (m, 2H, Hβ-Py), 8.55 (m, 2H, Hα-Py), 8.67 (br. s, 1H, 1′H); 20.8, 54.3, 116.6 (JCF = 22.5), 122.1, 125.4, 129.1 (JCF = 4.4), 130.1 (JCF = 8.2), 130.5 (JCF = 13.0), 141.2, 151.3, 161.9 (JCF = 245.9), 166.4 (C O) |
| 2j | C14H14FN3O | 95 | Oil | 1.37 (d J = 6.6, 3H, CH3), 4.20 (q J = 6.6, 1H, CH), 4.88 (br. s, 1H, 2′H), 6.90–7.24 (m, 4H, Ph), 7.49 (m, 2H, Hβ-Py); 8.52 (m, 2H, Hα-Py), 8.84 (br. s, 1H, 1′H); 22.4, 60.7, 114.9 (JCF = 21.7), 115.6 (JCF = 21.3), 122.1, 124.1, 131.1 (JCF = 8.1), 141.2, 146.7 (JCF = 6.9), 151.2, 163.9 (JCF = 245.5), 166.6 (C O) |
| 3bb | C14H14BFN4O | 88 | 148–150 (THF-hexane) | 2.67 (br. s, 2H, BH2), 4.06 (s, 2H, CH2), 5.81 (br. s, 1H, 2′H), 7.12–7.50 (m, 4H, Ph), 8.11 (m, 2H, Hβ-Py), 8.83 (m, 2H, Hα-Py), 10.70 (br. s, 1H, 1′H); 47.6, 115.2 (JCF = 50.7), 124.3 (JCF = 8.0), 124.7, 125.0, 129.4 (JCF = 19.2), 131.3, 144.9, 148.1, 160.8 (JCF = 244.9), 161.6 (C O) |
| 3cb | C14H14BFN4O | 89 | 107–108 (THF-hexane) | 2.68 (br. s, 2H, BH2), 4.03 (s, 2H, CH2), 5.90 (br. s, 1H, 2′H), 7.07–7.36 (m, 4H, Ph), 8.10 (m, 2H, Hβ-Py), 8.82 (m, 2H, Hα-Py), 10.65 (br. s, 1H, 1′H); 54.5, 114.6, 115.8, 125.7, 131.0, 140.4, 142.4, 145.7, 149.0, 161.5, 162.4 (C O) |
J values are expressed in hertz.
Dimethyl-d6 sulfoxide solution.
Melting points were recorded on a Kofler hot-stage apparatus and are uncorrected. NMR spectra were recorded on a Varian 300 MHz spectrometer; chemical shifts are given in δ units relative to the internal standard tetramethylsilane and refer to chloroform-d (CDCl3) or dimethyl-d6 sulfoxide solutions. Infrared spectra were recorded as Nujol mulls in the range of 4,000 to 300 cm−1 on a Perkin-Elmer 683 spectrophotometer. Microanalyses (C, H, and N) were obtained using a Carlo Erba model 1106 analyzer and are within ±0.4% of theoretical values.
In vitro antimycobacterial assays.
The in vitro antimycobacterial activity was assayed by the Tuberculosis Antimicrobial Acquisition Coordinating Facility (TAACF) antituberculosis drug discovery program, coordinated by the Southern Research Institute (Birmingham, Ala.) under the direction of the National Institute of Allergy and Infectious Diseases.
All compounds were initially screened against M. tuberculosis H37Rv (ATCC 27294) (American Type Culture Collection, Manassas, Va.) at the single concentration of 12.5 μg/ml in BACTEC 12B medium using a broth microdilution assay, the microplate Alamar blue assay (MABA) (9). Compounds exhibiting fluorescence were tested in the BACTEC 460 radiometric system (6). Compounds effecting <90% inhibition in the primary screen (i.e., MIC of >12.5 μg/ml) were not generally evaluated further. Compounds demonstrating at least 90% inhibition in the primary screen were retested at lower concentrations by serial dilution against M. tuberculosis H37Rv to determine the actual MIC, using the MABA. The MIC is defined as the lowest concentration effecting a reduction in fluorescence of 90% relative to controls. Rifampin was used as a reference drug.
Concurrent with the determination of MICs, compounds were tested for cytotoxicity (50% inhibitory concentration [IC50]) in Vero cells. After 72 h of exposure, viability was assessed on the basis of cellular conversion of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide into a formazan product using the Promega Celltiter 96 nonradioactive cell proliferation assay. The selectivity index (SI) is defined as the ratio of the measured IC50 in Vero cells to the MIC described above. The activities of compounds with SIs of >10 were confirmed in the BACTEC 460 system at 6.25 μg/ml.
Compounds were then tested for killing of M. tuberculosis Erdman (ATCC 35801) in monolayers of mouse bone marrow macrophages, as previously described (26), at fourfold concentrations equivalent to 0.25, 1, 4, and 16 times the MIC. The 90% effective concentration (EC90) and EC99 are the lowest concentrations effecting 90 and 99% reductions in CFU, respectively, at 7 days compared to drug-free controls.
Concurrently with this test, MICs against a panel of SDR strains (i.e., each strain being resistant to a single TB drug), typically an M. tuberculosis strain resistant to INH (ATCC 35822), a strain resistant to rifampin (ATCC 35838), and one or more additional SDR strains (chosen on the basis of compound type), were determined in the MABA system. The minimum bactericidal concentration (MBC) was then determined for M. tuberculosis H37Rv and for the appropriate drug-resistant strain by subculturing onto drug-free solid medium and enumeration of CFU after exposure in Middlebrook 7H9 medium supplemented with drug concentrations equivalent to and higher than the previously determined MICs against the respective strains.
RESULTS AND DISCUSSION
2′-Arylalkylisonicotinohydrazides (compounds 2) and their cyanoborane adducts (compounds 3) were prepared in good yields by the reaction of ISNEs (compounds 1) with sodium cyanoborohydride (NaBH3CN), according to previously described procedures (21) (Fig. 1). Variable-temperature NMR experiments in methyl-d3 alcohol-d solution showed that the cleavage of the PyN-B bond, and thus the conversion of compounds 3 to compounds 2, takes place at 80°C. However, when compounds 3 were kept for 7 to 10 days in phosphate buffer (pH 6.5 to 6.8) at 37°C, 1 H NMR monitoring did not reveal any alteration.
Isonicotinohydrazides (compounds 2) and cyanoboranes (compounds 3) were tested for in vitro antitubercular activity, in comparison with their parent ISNEs (compounds 1), according to the TAACF screening program, which is aimed to support the discovery of new agents for the treatment of TB. In this extended evaluation, compounds that in the primary screening (a high-throughput in vitro assay at 12.5 μg/ml) appear to be potentially of interest are selected for confirmatory and advanced testing so that, step by step, a comprehensive profile of the antimycobacterial activity of the new agent is provided.
Most of the tested compounds, assayed at 12.5 μg/ml, inhibited growth of M. tuberculosis H37Rv (ATCC 27294, susceptible to INH and rifampin) by 98 to 100%. They were then selected for further steps of the TAACF screening. On the other hand, cyanoboranes 3i, 3j, 3k, 3l, 3m, and 3o, which exhibited MICs of >12.5 μg/ml, were considered ineffective, and their screening was stopped. Selected derivatives 1 to 3 were retested at lower concentrations against M. tuberculosis H37Rv and M. tuberculosis Erdman (ATCC 35801, drug sensitive) to determine the actual MICs.
MICs and in vitro cytotoxicity to Vero cells, along with SIs calculated as IC50/MICH37Rv ratio, are reported in Table 2. Isonicotinohydrazides (compounds 2) displayed good activity against H37Rv, with MICs generally ranging between 0.2 and 1.6 μg/ml. In particular, the inhibitory effects of compounds 2a, 2b, and 2e (MICs = 0.2 μg/ml) were similar to those of rifampin. Slightly higher MICs were generally observed against M. tuberculosis Erdman. Compared to the corresponding ISNEs (compounds 1), however, hydrazides (compounds 2) were 2- to 16-fold less active against both the H37Rv and Erdman strains. The presence of the cyanoborane moiety (BH2CN) in compounds 3 brought about a remarkable decrease in activity. The results summarized in Table 2 clearly show that the antimycobacterial effectiveness against both mycobacteria decreased in the order ISNEs (compounds 1) > isonicotinohydrazides (compounds 2) > cyanoboranes (compounds 3).
TABLE 2.
In vitro antimycobacterial activities against M. tuberculosis H37Rv and M. tuberculosis Erdman and cytotoxicities
| Compound | % Inhibition of H37Rv | MIC (μg/ml)a for: |
IC50 (μg/ml) (Vero cells) | SIb | |
|---|---|---|---|---|---|
| H37Rv | Erdman | ||||
| 1bc | 100 | 0.05 | 0.05 | 8.4 | 168 |
| 1cc | 99 | 0.05 | 0.4 | >6.25 | >125 |
| 1ec | 99 | 0.1 | 0.2 | >10 | >100 |
| 1h | 99 | 0.1 | 0.1 | >125 | >1,250 |
| 1ic | 100 | 0.2 | 0.39 | >125 | >625 |
| 1jc | 100 | <0.05 | |||
| 1kc | 99 | 0.05 | 0.2 | >10 | >200 |
| 1l | 99 | 0.1 | |||
| 1nc | 100 | 0.1 | |||
| 1pc | 100 | 0.05 | |||
| 2a | 97 | 0.2 | 0.39 | >10 | >50 |
| 2b | 97 | 0.2 | 0.78 | 125 | 625 |
| 2c | 98 | 0.39 | |||
| 2d | 98 | 0.39 | >125 | >320.5 | |
| 2e | 98 | 0.2 | >6.25 | >10 | >50 |
| 2f | 98 | 1.6 | 63.8 | 39.9 | |
| 2h | 98 | 6.25 | 25 | >125 | >20 |
| 2i | 98 | 1.6 | 3.13 | 125 | 78.12 |
| 2j | 98 | 1.6 | |||
| 2k | 98 | 1.6 | 1.56 | >125 | >78 |
| 2l | 98 | 1.6 | 3.13 | 106 | 66.2 |
| 2m | 98 | 3.13 | 46.5 | 14.9 | |
| 2n | 98 | 1.6 | 3.13 | 63.8 | 39.9 |
| 2o | 95 | 12.5 | >125 | >9.7 | |
| 2p | 98 | 0.39 | 0.78 | 8.5 | 21.8 |
| 3a | 98 | 0.8 | >10 | >12.5 | |
| 3b | 96 | 3.13 | >10 | >3.2 | |
| 3c | 97 | 0.8 | 6.7 | 8.4 | |
| 3e | 98 | 0.8 | |||
| 3g | 98 | 0.39 | |||
| 3h | 95 | 12.5 | >10 | >0.8 | |
| 3n | 93 | >6.25 | |||
| 3p | 96 | 0.78 | 95 | 122 | |
| INH | 99 | 0.025–0.05 | >1,000 | >20,000 | |
| Rifampin | 99 | 0.06–0.25 | >100 | >400 | |
The MIC is defined as the lowest concentration inhibiting 90% of the inoculum relative to controls.
IC50/MICH37Rv ratio.
Primary antimycobacterial screening reported in reference 2.
Fluorine and trifluoromethyl substitutions on the benzene ring appeared to be the most beneficial for activity; in addition, the trifluoromethyl group directly linked to an iminic carbon (as in compound 1p) or to a 2′-methylene group (as in compounds 2p and 3p) gave high MICs. The replacement of the arylmethyl moiety (R = H) with the arylethyl one (R = CH3) in compounds 2 and 3 was not beneficial, as demonstrated by the a-h, b-i, c-j, d-k, and e-l pairs of compounds.
The in vitro cytotoxicity to Vero cells was shown to decrease in the order cyanoboranes (compounds 3) > ISNEs (compounds 1) ≥ hydrazides (compounds 2) (Table 2). Hydrazides (compounds 2), on the whole, are the least cytotoxic among the tested compounds, although their cytotoxicity is higher than that of INH. However, they showed a good differential between active and cytotoxic doses, as the SIs indicate; particularly indicative examples are compounds 2b, with an SI of 625, and 2d, with an SI of >320.5. On the other hand, cyanoboranes (compounds 3) showed the lowest SIs among the tested compounds, with the exception of compound 3p, which is endowed with good activity and a good SI (122). On the whole, the presence of the BH2CN moiety appeared to be detrimental, in that cytotoxicity was enhanced while antimycobacterial activity was lowered. Such a feature of compounds 3 could be related to their antiproliferative properties against several human tumor cell lines, which we previously reported (31).
In the subsequent steps of the TAACF program, compounds with SIs of >10 were tested for killing of M. tuberculosis Erdman (ATCC 35801) in monolayers of mouse bone marrow macrophages (26). Such an assay must be considered highly predictive for an effective antitubercular agent, since M. tuberculosis is an intracellular parasite, living and multiplying inside macrophages; thus, many factors that are not reflected in a broth culture assay could influence the activity of a drug within the infected host cell.
Interestingly, the selected compounds were very active in this model (Table 3). Indeed, their EC90s (and often their EC99s also) were much lower than the corresponding MICs against both the Erdman and H37Rv strains (Tables 2 and 3); thus, EC90/MIC ratios were considerably lower than the stated activity criterion, which is 16, especially in the cases of compounds 1c, 2a, 2d, 2e, and 2h. The most potent derivative was 2e, which was able to kill M. tuberculosis Erdman growing within macrophages at a concentration at least 1,250-fold lower than the MIC observed in the culture medium. The activities of compounds 2a and 2h were also appreciable, since their EC90s were 78-fold lower than their MICs. Moreover, compounds 1c, 2a, 2d, and 2e showed EC90s lower than those of INH and rifampin (Table 3).
TABLE 3.
In vitro antimycobacterial activities against M. tuberculosis Erdman in monolayers of TB-infected mouse bone marrow macrophages
| Compound | EC90 (μg/ml)a | EC99 (μg/ml)a | EC90/MICErdmanb | EC90/MICH37Rvb |
|---|---|---|---|---|
| 1c | 0.011 | 0.043 | 0.0275 | 0.22 |
| 1e | 0.047 | 0.15 | 0.235 | 0.47 |
| 1h | 0.074 | 0.23 | 0.74 | |
| 1i | 0.081 | 0.36 | 0.21 | 0.41 |
| 1k | 0.038 | 0.555 | 0.19 | 0.76 |
| 2a | 0.005 | 0.166 | 0.013 | 0.025 |
| 2b | 0.28 | 1.67 | 0.36 | 1.4 |
| 2d | 0.014 | 5.08 | 0.04 | |
| 2e | 0.005 | 0.228 | <0.0008 | 0.025 |
| 2h | 0.317 | 1.72 | 0.013 | 0.051 |
| 2k | 1.84 | 5.25 | 1.18 | 1.15 |
| 2m | 1.38 | 4.09 | 0.44 | |
| 2p | 0.14 | 0.71 | 0.18 | 0.36 |
| 3a | 0.116 | 1.49 | 0.145 | |
| 3p | 1.14 | 10.58 | 1.46 | |
| INH | 0.03 | 0.42 | 0.6–1.2 | |
| Rifampin | 0.04–0.1 | 0.5–1.5 | 0.16–1.67 |
EC90 and EC99 are defined as the concentrations effecting 90 and 99% reductions, respectively, in CFU at 7 days compared to drug-free controls.
Compounds with EC90/MIC ratios of <16 are considered active.
These data clearly indicate that the tested compounds are active against both intracellular and extracellular M. tuberculosis strains. Furthermore, they are even more effective in killing bacilli growing within cells than those growing in culture media.
Concurrently with testing of the compounds in macrophages, evaluation of activity against SDR M. tuberculosis strains was performed (Table 4). Several of the compounds 1 to 3 displayed moderate to high activity against rifampin-, ethambutol-, and kanamycin-resistant M. tuberculosis; MICs ranged between 0.05 and 3.13 μg/ml, with the exception of compound 2h. The susceptibilities of these strains can be considered comparable to that of H37Rv, as was indicated by the ratios of MICs against resistant and nonresistant strains, which were generally about 1.
TABLE 4.
In vitro antimycobacterial activities against SDR M. tuberculosis strains
| Compound | MIC (μg/ml)a for: |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| INH-R | INH-R/H37Rv | RMP-R | RMP-R/H37Rv | EMB-R | EMB-R/H37Rv | KM-R | KM-R/H37Rv | CIP-R | CIP-R/H37Rv | ETA-R | ETA-R/H37Rv | TAC-R | TAC-R/H37Rv | |
| 1b | >1.6 | >32 | 0.05 | 1.00 | 0.05 | 1.00 | NTb | NT | >1.6 | >32 | >1.6 | >32 | ||
| 1c | >6.4 | >128 | ≤0.1 | ≤2 | ≤0.1 | ≤2 | ≤0.1 | ≤2 | 0.2 | 4.00 | NT | NT | ||
| 1e | >3.2 | >32 | 0.1 | 1.00 | 0.1 | 1.00 | 0.1 | 1.00 | 0.39 | 3.90 | NT | NT | ||
| 1h | >3.2 | >32 | 0.2 | 2.00 | 0.2 | 2.00 | NT | NT | 3.2 | 32 | >3.2 | >32 | ||
| 1i | >6.25 | >31.25 | 0.2 | 1.00 | 0.2 | 1.00 | NT | NT | >6.25 | >31.25 | >6.25 | >31.25 | ||
| 1k | >1.6 | >32 | 0.2 | 4.00 | 0.1 | 2.00 | NT | NT | >1.6 | >32 | >1.6 | >32 | ||
| 2a | >6.25 | >31.25 | 0.39 | 1.95 | 0.2 | 1.00 | NT | NT | >6.25 | >31.25 | >6.25 | >31.25 | ||
| 2b | >6.4 | >32 | 0.39 | 1.95 | 0.39 | 1.95 | 0.4 | 2.00 | 0.78 | 3.90 | NT | NT | ||
| 2e | >6.25 | >31.25 | 0.39 | 1.95 | 0.39 | 1.95 | NT | NT | >6.25 | >31.25 | >6.25 | >31.25 | ||
| 2h | >200 | >32 | 12.5 | 2.00 | 6.25 | 1.00 | 6.25 | 1.00 | 25 | 4.00 | NT | NT | ||
| 2i | >50 | >31.25 | 3.13 | 1.96 | 1.56 | 0.98 | 3.13 | 1.96 | 6.26 | 3.91 | NT | NT | ||
| 2k | >50 | >31.25 | 3.13 | 1.96 | ≤0.78 | ≤0.49 | NT | NT | >50 | >31.25 | >50 | >31.25 | ||
| 2l | >50 | >31.25 | 3.13 | 1.96 | ≤0.78 | ≤0.49 | NT | NT | >50 | >31.25 | >50 | >31.25 | ||
| 2n | >50 | >31.25 | 3.13 | 1.96 | ≤0.78 | ≤0.49 | NT | NT | >50 | >31.25 | 50 | 31.25 | ||
| 2p | >12.5 | >32.05 | 0.78 | 2.00 | 0.39 | 1.00 | NT | NT | >12.5 | >32.05 | >12.5 | >32.05 | ||
INH-R, INH-resistant strain; RMP-R, rifampin-resistant strain; EMB-R, ethambutol-resistant strain; KM-R, kanamycin-resistant strain; CIP-R, ciprofloxacin-resistant strain; ETA-R, ethionamide-resistant strain; TAC-R, thiacetazone-resistant strain. Ratios of MICs against resistant and nonresistant strains of ≅1 indicate activity also against the resistant strain; larger ratios indicate cross-resistance.
NT, not tested.
The MBCs of some compounds against H37Rv and rifampin- and INH-resistant strains were also determined (Table 5). Most of the selected compounds showed rather low MBCs against the rifampin-resistant strain, ranging between 0.0125 and 3.13 μg/ml. As expected for INH derivatives, compounds 1 to 3 were ineffective against the INH-resistant strain, as indicated by the high ratios of MICs against INH-resistant and -susceptible strains, which are indicative of the presence of cross-resistance. Cross-resistance between compounds 1 to 3 and ethionamide, as well as thiacetazone, was also observed (Table 4). These findings, as well as the structural analogy, suggested that these INH derivatives should act at the same target as the parental drug. In fact, cross-resistance between INH and ethionamide and partial cross-resistance between ethionamide and thiacetazone are well known (1, 13, 22, 25). Although INH resistance is a complex and multifactorial phenomenon also involving alterations of the katG gene, coding for catalase-peroxidase production (4, 19, 32, 33), mutations within the mycobacterial inhA gene can confer resistance to both INH and ethionamide, with inhA being a primary target of action of both INH and ethionamide (1, 13, 23, 24).
TABLE 5.
MBCs against H37Rv and SDR strains
| Compound | MIC (μg/ml)a for: |
||
|---|---|---|---|
| H37Rv | RMP-R | INH-R | |
| 1b | 0.4 | 0.4 | >1.6 |
| 1c | 0.8 | 0.0125 | >1.6 |
| 1e | >3.2 | 0.05 | >3.2 |
| 1i | 1.56 | 0.39 | >6.25 |
| 1k | 0.2 | >1.6 | >1.6 |
| 2a | 3.13 | 1.56 | >6.25 |
| 2b | >6.25 | 0.39 | >6.25 |
| 2h | 50 | 3.13 | >200 |
| 2i | >50 | 3.13 | >50 |
| 2k | 25 | 12.5 | >50 |
| 2l | 25 | 12.5 | >50 |
| 2n | 25 | 12.5 | >50 |
| 2p | 6.25 | 3.13 | >12.5 |
| INH | 0.1 | >1.6 | |
RMP-R, rifampin-resistant strain; INH-R, INH-resistant strain.
In conclusion, an extended evaluation of the in vitro antimycobacterial activities of isonicotinohydrazides (compounds 2), cyanoborane adducts (compounds 3), and parental ISNEs (compounds 1) was performed. Most of them, particularly ISNEs and hydrazides, displayed good antimycobacterial properties along with rather low cytotoxicity and proved to be interesting for at least two beneficial aspects. First, they were effective in killing M. tuberculosis growing within macrophages at concentrations much lower than the corresponding MICs in culture media (1,250-fold lower in the case of compound 2e); this finding is of interest because mycobacteria existing in cells are difficult to treat. Second, several hydrazides and ISNEs also displayed good inhibitory activity against ethambutol-, kanamycin-, and rifampin-resistant strains. In particular, isonicotinohydrazides 2a, 2d, and 2e showed the most appreciable in vitro activity, being more effective than parental INH in the macrophage model.
Acknowledgments
We gratefully acknowledge the staff of the Tuberculosis Antimicrobial Acquisition and Coordinating Facility (TAACF), coordinated by the Southern Research Institute (Birmingham, Ala.) under the direction of the U.S. National Institute of Allergy and Infectious Diseases. We also thank Giuseppe Teti (University of Messina, Messina, Italy) for his helpful suggestions.
This work was supported by financial assistance from Ministero Università Ricerca Scientifica e Tecnologica (MURST, Rome) and from Consiglio Nazionale Ricerche (CNR, Italy).
REFERENCES
- 1.Banerjee, A., E. Dubnau, A. Quemard, V. Balasubramanian, K. S. Um, T. Wilson, D. Collins, G. DeLisle, and W. R. Jacobs. 1994. inhA, a gene encoding a target for isoniazid and ethionamide in Mycobacterium tuberculosis. Science 263:227–230. [DOI] [PubMed] [Google Scholar]
- 2.Bottari, B., R. Maccari, F. Monforte, R. Ottanà, E. Rotondo, and M. G. Vigorita. 2000. Isoniazid-related copper(II) and nickel(II) complexes with antimycobacterial in vitro activity. Bioorg. Med. Chem. Lett. 10:657–660. [DOI] [PubMed] [Google Scholar]
- 3.Bottari, B., R. Maccari, F. Monforte, R. Ottanà, E. Rotondo, and M. G. Vigorita. 2001. Antimycobacterial in vitro activity of cobalt(II) isonicotinoylhydrazone complexes. Bioorg. Med. Chem. Lett. 11:301–303. [DOI] [PubMed] [Google Scholar]
- 4.F. R. Cockerill, J. R. Uhl, Z. Temesgen, Y. Zhang, L. Stockman, G. D. Roberts, D. L. Williams, and B. C. Kline. 1995. Rapid identification of a point mutation of the Mycobacterium tuberculosis catalase-peroxidase (katG) gene associated with isoniazid resistance. J. Infect. Dis. 171:240–245. [DOI] [PubMed] [Google Scholar]
- 5.Collins, F. M. 1989. Mycobacterial disease, immunosuppression, and acquired immunodeficiency syndrome. Clin. Microbiol. Rev. 2:360–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins, L. A., and S. G. Franzblau. 1997. Microplate Alamar blue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob. Agents Chemother. 41:1004–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dessen, A., A. Quémard, J. S. Blanchard, W. R. Jacobs, Jr., and J. C. Sacchettini. 1995. Crystal structure and function of the isoniazid target of Mycobacterium tuberculosis. Science 267:1638–1641. [DOI] [PubMed] [Google Scholar]
- 8.Fox, H. H., and J. T. Gibas. 1955. Synthetic tuberculostats. IX. Dialkyl derivatives of isonicotinylhydrazine. J. Org. Chem. 20:60–69. [Google Scholar]
- 9.Franzblau, S. G., R. S. Witzig, J. C. McLaughlin, P. Torres, G. Madico, A. Hernandez, M. T. Degnan, M. B. Cook, V. K. Quenzer, R. M. Freguson, and R. H. Gilman. 1998. Rapid, low-technology MIC determination with clinical Mycobacterium tuberculosis isolates by using the microplate Alamar blue assay. J. Clin. Microbiol. 36:362–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graham, N. M. H., N. Galai, K. E. Nelson, J. Astemborski, M. Bonds, R. T. Rizzo, L. Sheeley, and D. Vlahov. 1996. Effect of isoniazid chemoprophylaxis on HIV-related mycobacterial disease. Arch. Intern. Med. 156:889–894. [PubMed] [Google Scholar]
- 11.Halsey, N. A., J. S. Coberly, J. Desormeaux, P. Losikoff, J. Atkinson, L. H. Moulton, M. Contave, M. Johnson, H. Davis, L. Geiter, E. Johnson, R. Huebner, R. Boulos, and R. E. Chaisson. 1998. Randomised trial of isoniazid versus rifampicin and pyrazinamide for prevention of tuberculosis in HIV-1 infection. Lancet 351:786–792. [DOI] [PubMed] [Google Scholar]
- 12.Inderlied, C. B., C. A. Kemper, and L. E. M. Bermudez. 1993. The Mycobacterium avium complex. Clin. Microbiol. Rev. 6:266–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnsson, K., D. S. King, and P. G. Schultz. 1995. Studies on the mechanism of action of isoniazid and ethionamide in the chemotherapy of tuberculosis. J. Am. Chem. Soc. 117:5009–5010. [Google Scholar]
- 14.Lewis, A., and R. G. Shepherd. 1970. Antimycobacterial agents, p.409–491 In A. Burger (ed.), Medicinal chemistry, 3rd ed. Wiley-Interscience, New York, N.Y.
- 15.Mandell, G. L., and W. A. Petri, Jr. 1996. Antimicrobial agents used in the chemotherapy of tuberculosis, p.1155–1174. In Goodman and Gilman’s the pharmacological basis of therapeutics, 9th ed. McGraw-Hill, New York, N.Y.
- 16.McMillan, F. H., F. Leonard, R. I. Meltzer, and J. A. King. 1953. Antitubercular substances. II. Substitution products of isonicotinic hydrazide. J. Am. Pharm. Assoc. 42:457–464. [DOI] [PubMed] [Google Scholar]
- 17.Mdluli, K., J. Swanson, E. Fischer, R. E. Lee, and C. E. Barry. 1998. Mechanisms involved in the intrinsic isoniazid resistance of Mycobacterium avium. Mol. Microbiol. 27:1223–1233. [DOI] [PubMed] [Google Scholar]
- 18.Mikusová, K., M. Mikus, G. S. Besra, I. Hancock, and P. J. Brennan. 1996. Biosynthesis of the linkage region of the mycobacterial cell wall. J. Biol. Chem. 271:7820–7828. [DOI] [PubMed] [Google Scholar]
- 19.Morris, S., G. H. Bai, P. Suffys, L. Portilo-Gomez, M. Fairchok, and D. Rouse. 1995. Molecular mechanisms of multiple drug resistance in clinical isolates of Mycobacterium tuberculosis. J. Infect. Dis. 171:954–960. [DOI] [PubMed] [Google Scholar]
- 20.Musser, J. M., V. Kapur, D. L. Williams, B. N. Kreiswirth, D. Soolingen, and J. D. A. Embden. 1996. Characterization of the catalase-peroxidase gene (katG) and inhA locus in isoniazid-resistant and -susceptible strains of Mycobacterium tuberculosis by automated DNA sequencing: restricted array of mutations associated with drug resistance. J. Infect. Dis. 173:196–202. [DOI] [PubMed] [Google Scholar]
- 21.Ottanà, R., R. Maccari, M. G. Vigorita, and E. Rotondo. 1998. Synthesis of mono- and di-cyanoborane adducts from isonicotinoylhydrazones and sodium cyanoborohydride. J. Chem. Res. (S)550–551, (M)2532–2544.
- 22.Quemard, A., A. Dessen, M. Sugantino, W. R. Jacobs, Jr., J. C. Sacchettini, and J. S. Blanchard. 1996. Binding of catalase-peroxidase-activated isoniazid to wild-type and mutant Mycobacterium tuberculosis enoyl-ACP reductases. J. Am. Chem. Soc. 118:1561–1562. [Google Scholar]
- 23.Rouse, D. A., Z. Li, G. Bai, and S. L. Morris. 1995. Characterization of the katG and inhA genes of isoniazid-resistant clinical isolates of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 39:2472–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rozwarski, D. A., G. A. Grant, D. H. R. Barton, W. R. Jacobs, Jr., and J. C. Sacchettini. 1998. Modification of the NADH of the isoniazid target (inhA) from Mycobacterium tuberculosis. Science 279:98–102. [DOI] [PubMed] [Google Scholar]
- 25.Sensi, P., and G. Gialdroni Grassi. 1996. Antimycobacterial agents, p.575–635. In M. E. Wolff (ed.), Burger’s medicinal chemistry and drug discovery, 5th ed. John Wiley & Sons, Inc., New York, N.Y.
- 26.Skinner, P. S., S. K. Furney, M. R. Jacobs, G. Klopman, J. J. Ellner, and I. M. Orme. 1994. A bone marrow-derived murine macrophage model for evaluating efficacy of antimycobacterial drugs under relevant physiological conditions. Antimicrob. Agents Chemother. 38:2557–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Telzak, E. E., K. Sepkowitz, P. Alpert, S. Mannheimer, F. Medard, W. El-Sadr, S. Blum, A. Gagliardi, N. Salomon, and G. Turett. 1995. Multidrug-resistant tuberculosis in patients without HIV infection. N. Engl. J. Med. 333:907–911. [DOI] [PubMed] [Google Scholar]
- 28.Vigorita, M. G., M. Basile, C. Zappalà, G. Gabbrielli, and F. Pizzimenti. 1992. Halogenated isoniazid derivatives as possible antitubercular and antineoplastic agents. Il Farmaco. 47:893–906. [PubMed] [Google Scholar]
- 29.Vigorita, M. G., R. Ottanà, C. Zappalà, R. Maccari, F. C. Pizzimenti, and G. Gabbrielli. 1994. Halogentaed isoniazid derivatives as possible antimycobacterial and anti-HIV agents. Il Farmaco. 49:775–781. [PubMed] [Google Scholar]
- 30.Vigorita, M. G., R. Ottanà, C. Zappalà, R. Maccari, F. C. Pizzimenti, and G. Gabbrielli. 1995. 2-(4-Pyridyl)-Δ2-1,3,4-oxadiazolines from isonicotinoylhydrazones and diazomethane as potential antimycobacterial and anti-HIV agents. Il Farmaco. 50:783–786. [PubMed] [Google Scholar]
- 31.Vigorita, M. G., R. Maccari, R. Ottanà, and F. Monforte. 1999. Lipophilic analogs of isoniazid with antiproliferative in vitro activity. Med. Chem. Res. 9:306–321. [Google Scholar]
- 32.Wilson, T. M., G. W. DeLisle, and D. M. Collins. 1995. Effect of inhA and katG on isoniazid resistance and virulence of Mycobacterium bovis. Mol. Microbiol. 15:1009–1015. [DOI] [PubMed] [Google Scholar]
- 33.Zhang, Y., B. Heym, B. Allen, D. Young, and S. Cole. 1992. The catalase-peroxidase gene and isoniazid resistance of Mycobacterium tuberculosis. Nature 358:591–593. [DOI] [PubMed] [Google Scholar]