Abstract
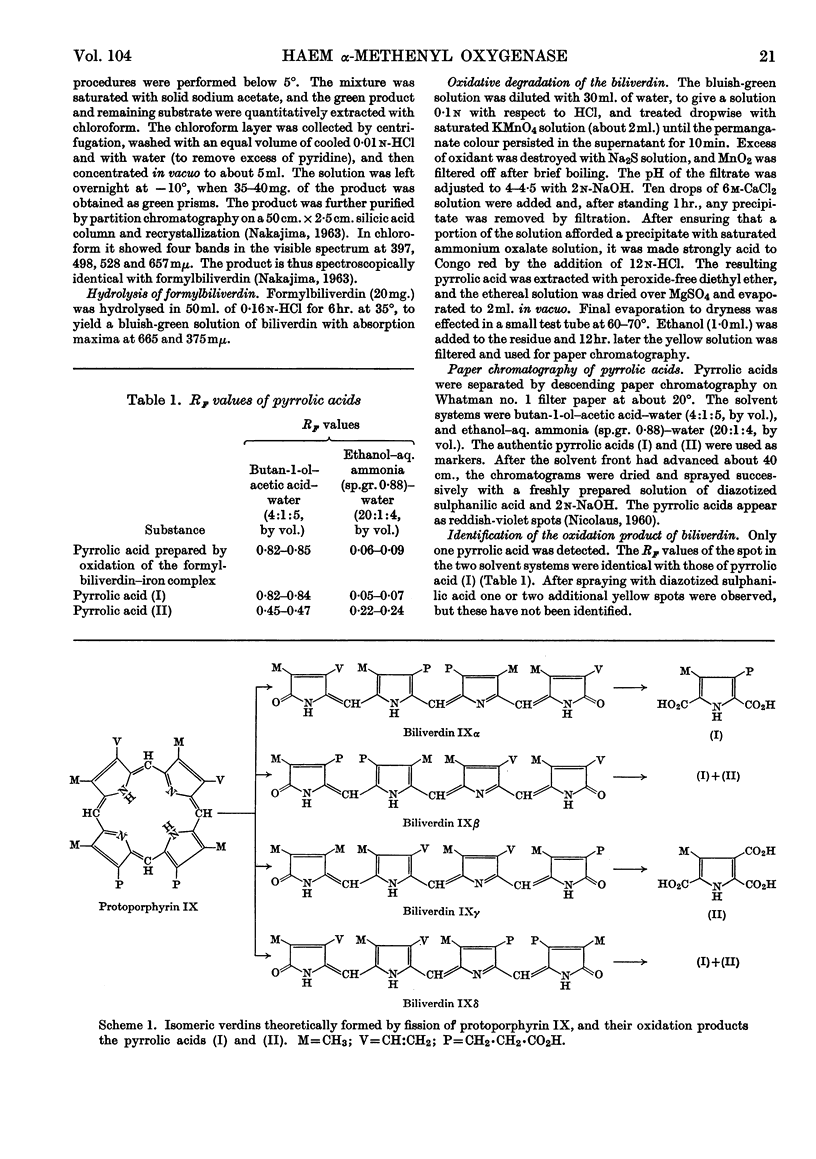
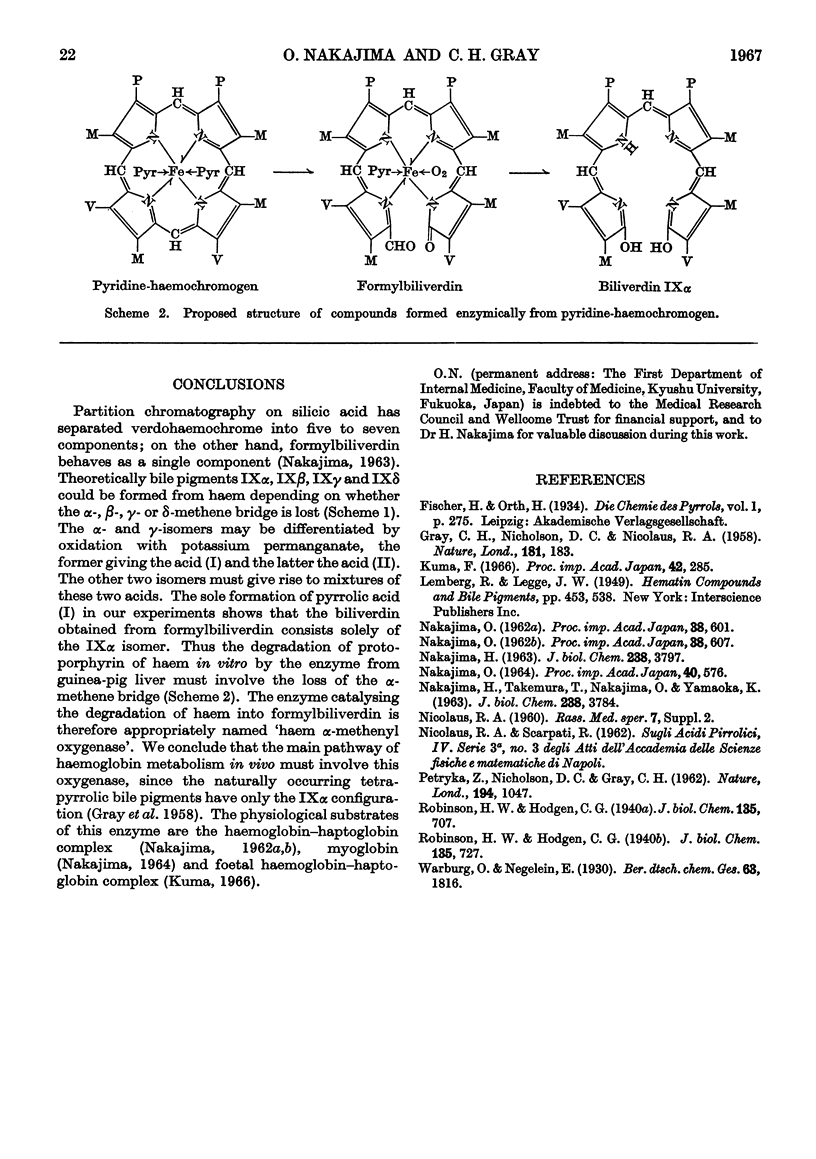
1. The enzyme from guinea-pig liver that degrades pyridine-haemochromogen into formylbiliverdin, a possible precursor of biliverdin, breaks the porphyrin ring exclusively at the α-methene bridge. The enzyme is therefore appropriately named haem α-methenyl oxygenase. 2. It is deduced that this enzyme plays an essential role in haemoglobin catabolism in vivo.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- GRAY C. H., NICHOLSON D. C., NICOLAUS R. A. The IX-alpha structure of the common bile pigments. Nature. 1958 Jan 18;181(4603):183–185. doi: 10.1038/181183b0. [DOI] [PubMed] [Google Scholar]
- NAKAJIMA H. STUDIES ON HEME ALPHA-METHENYL OXYGENASE. II. THE ISOLATION AND CHARACTERIZATION OF THE FINAL REACTION PRODUCT, A POSSIBLE PRECURSOR OF BILIVERDIN. J Biol Chem. 1963 Nov;238:3797–3801. [PubMed] [Google Scholar]
- NAKAJIMA H., TAKEMURA T., NAKAJIMA O., YAMAOKA K. STUDIES ON HEME ALPHA-METHENYL OXYGENASE. I. THE ENZYMATIC CONVERSION OF PYRIDINE-HEMICHROMOGEN AND HEMOGLOBIN-HAPTOGLOBIN INTO A POSSIBLE PRECURSOR OF BILIVERDIN. J Biol Chem. 1963 Nov;238:3784–3796. [PubMed] [Google Scholar]
- PETRYKA Z., NICHOLSON D. C., GRAY C. H. Isomeric bile pigments as products of the in vitro fission of haemin. Nature. 1962 Jun 16;194:1047–1048. doi: 10.1038/1941047a0. [DOI] [PubMed] [Google Scholar]



