Abstract
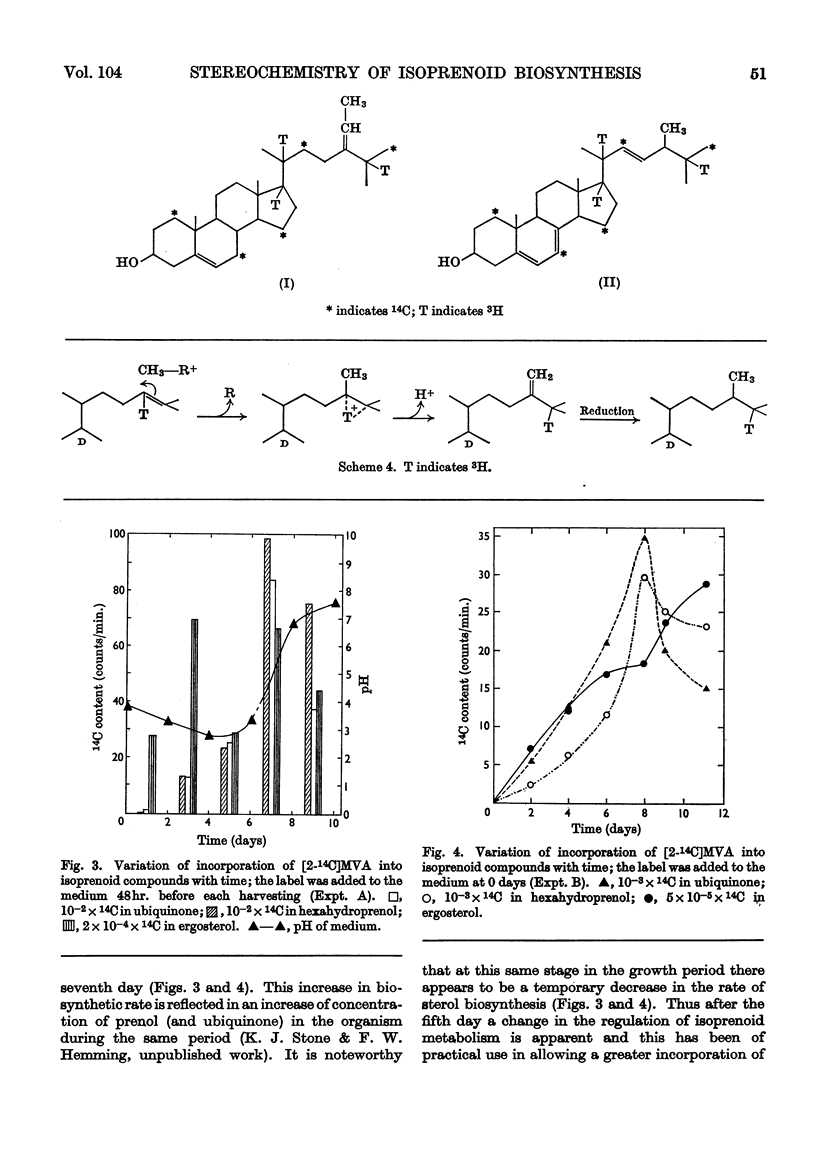
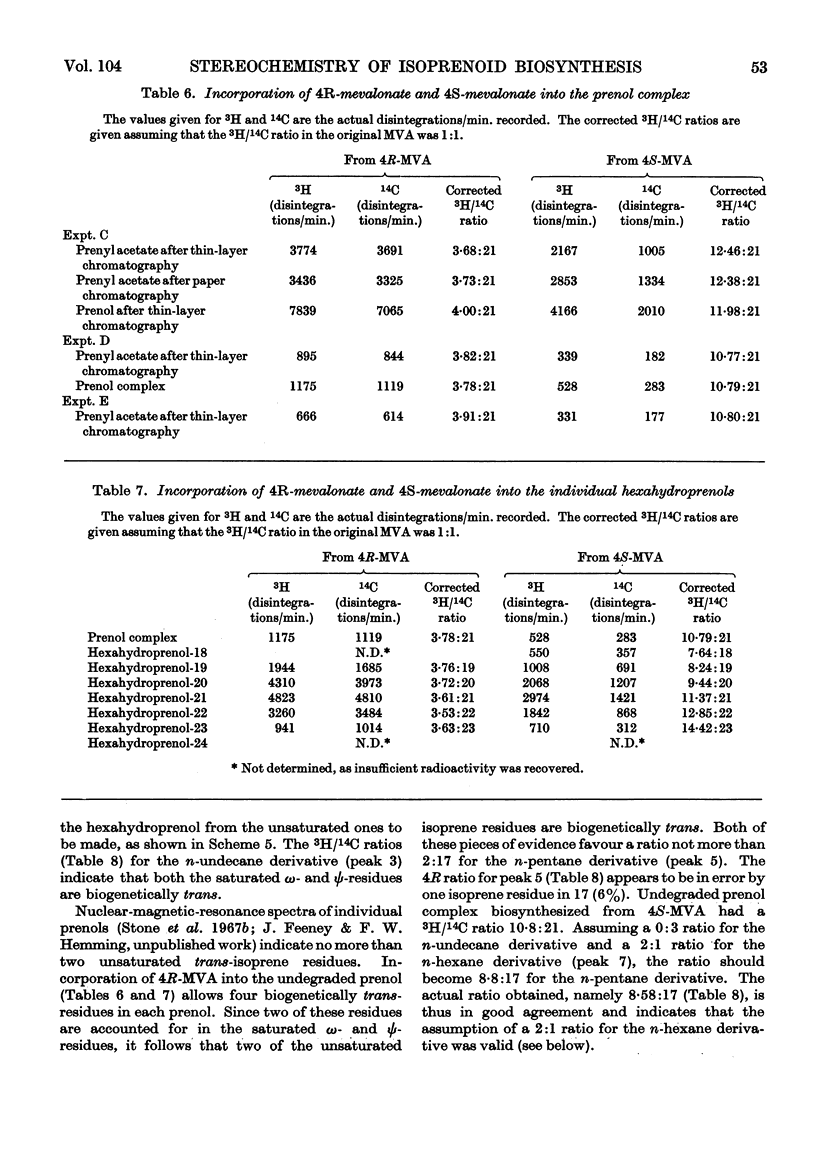
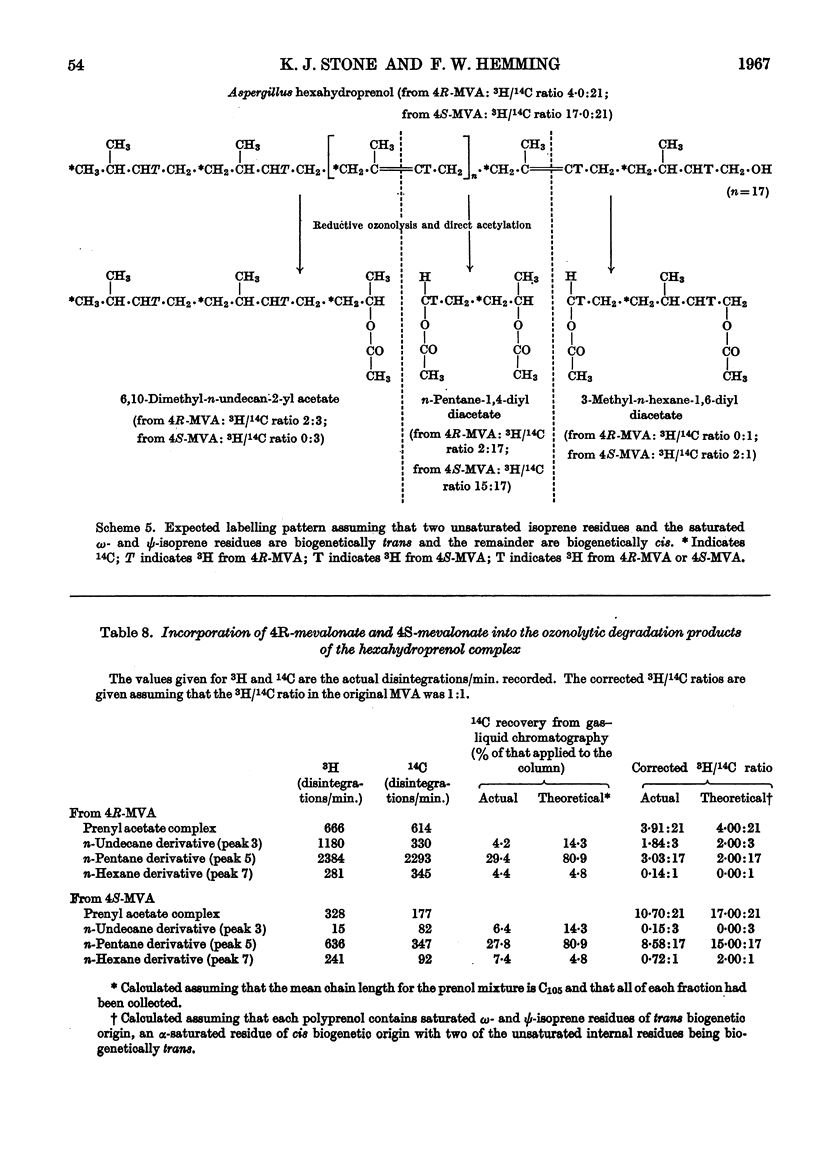
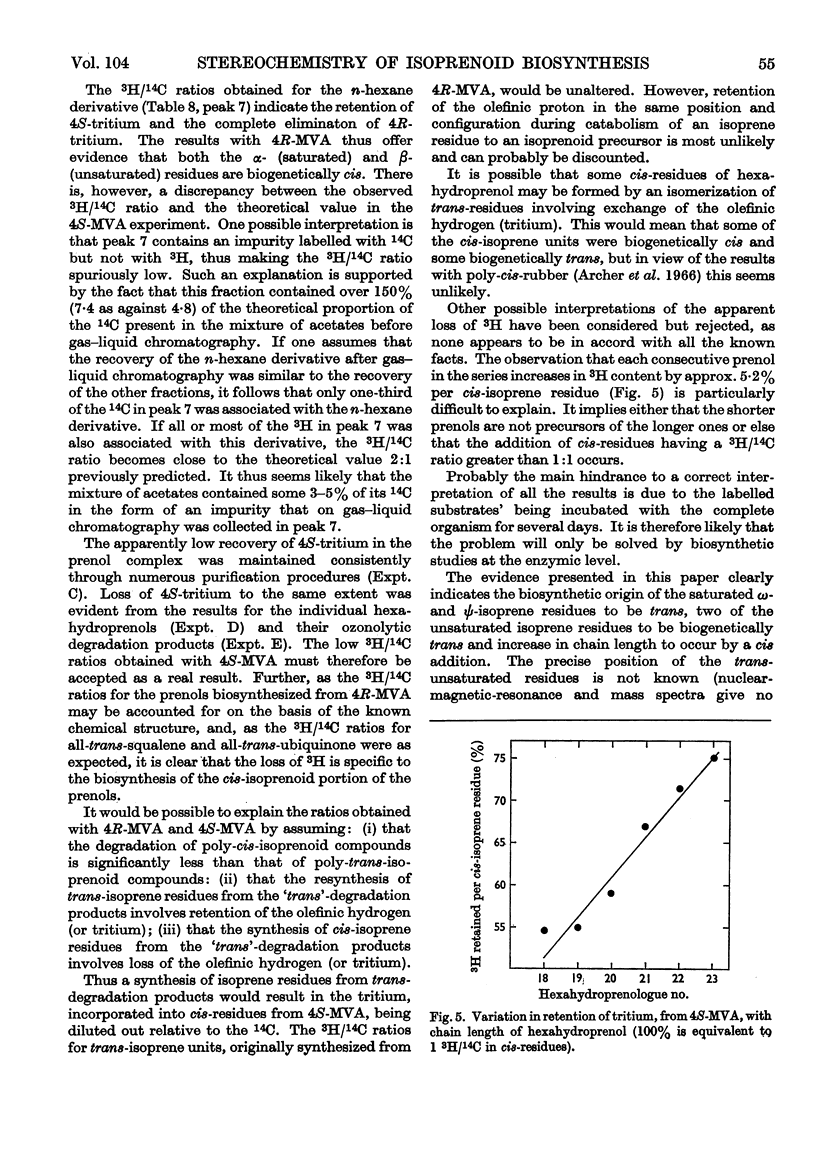
1. The mycelium of Aspergillus fumigatus has been shown to incorporate mevalonate into squalene, ubiquinone, ergosterol and hexahydroprenol. 2. The 3H/14C ratio in ubiquinone, biosynthesized from [2-14C-(4R)-4-3H1]mevalonate, is the same as in the squalene; essentially no 3H was incorporated from [2-14C-(4S)-4-3H1]mevalonate, indicating the biosynthesis of biogenetically trans-isoprene units. 3. The 3H/14C ratio for ergosterol (from `4R-mevalonate') was 3:5, showing that the proton at C-24 is not lost during alkylation of the side chain; it probably migrates to C-25. 4. As 3H from both mevalonates was incorporated into the hexahydroprenols the biosynthesis of both cis- and trans-isoprene units must occur. 5. The saturated ω- and ψ-isoprene units are shown to be biogenetically trans, as are two of the unsaturated residues. 6. The saturated α- and unsaturated β-isoprene residues are both biogenetically cis. 7. An inexplicable loss of approximately half of the olefinic protons from the cis-portion of hexahydroprenol occurs; possible reasons for this loss are discussed. 8. Increase in chain length of the hexahydroprenols is by a cis addition. 9. A biosynthesis of hexahydroprenols by addition of cis-isoprene units to all-trans-geranylgeranyl pyrophosphate, or a dihydro or tetrahydro derivative thereof, is suggested.
Full text
PDF

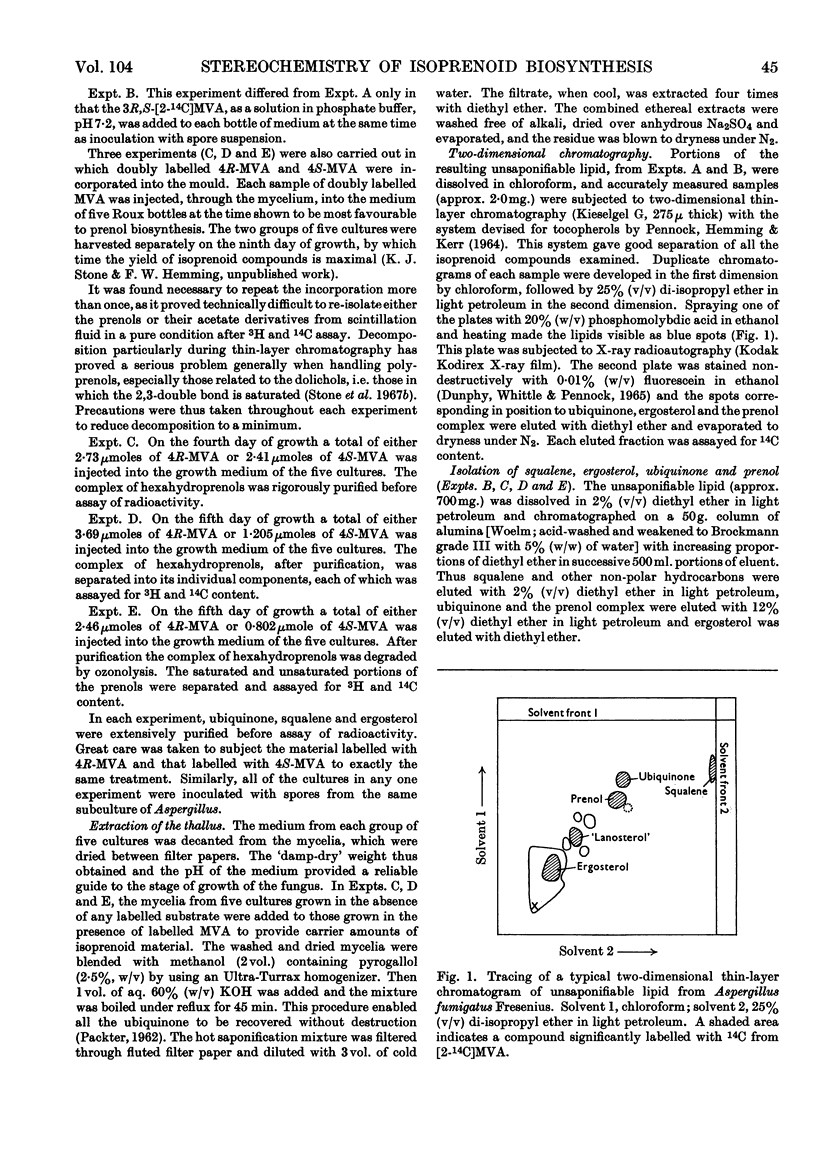


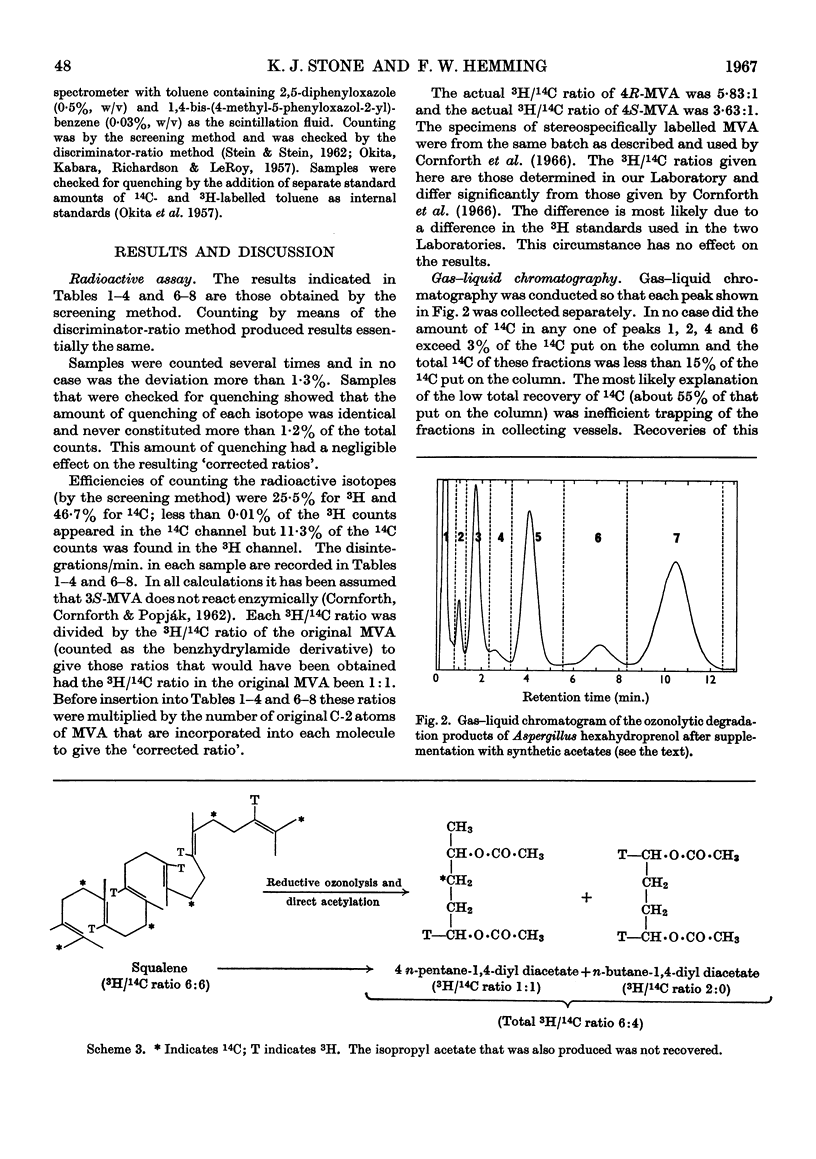
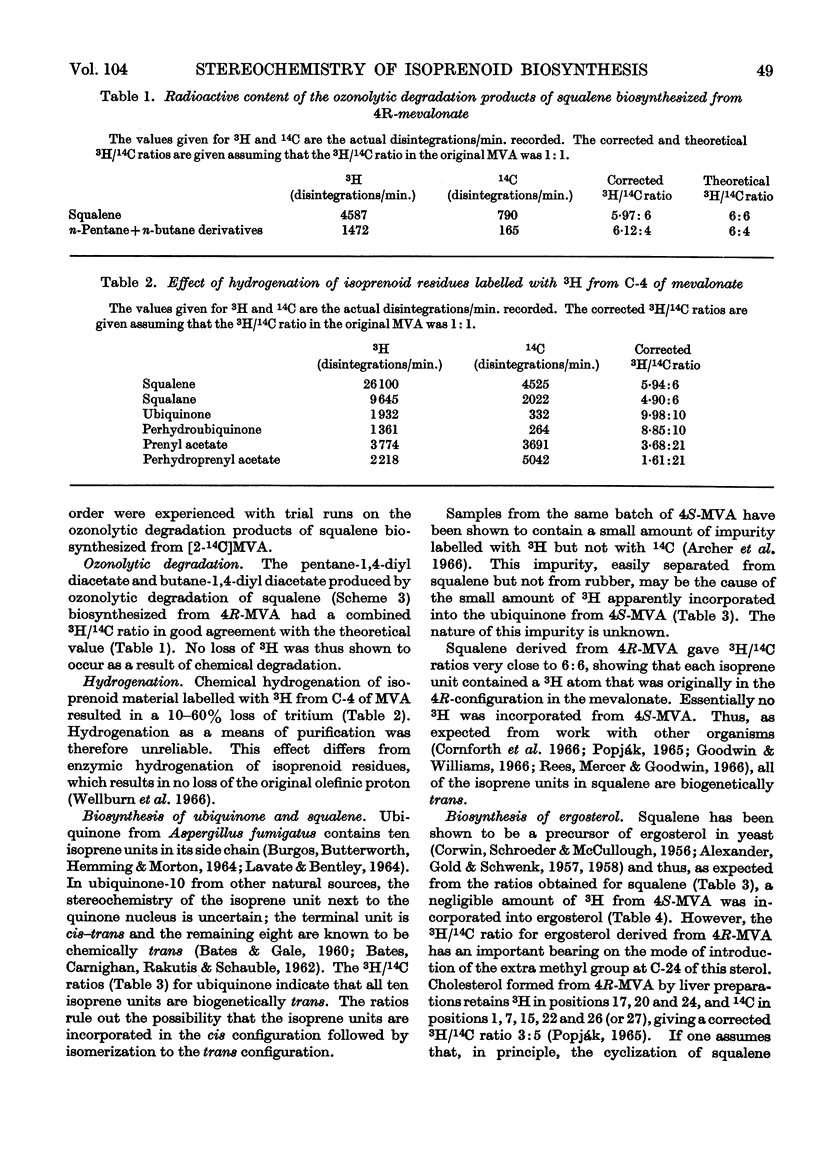
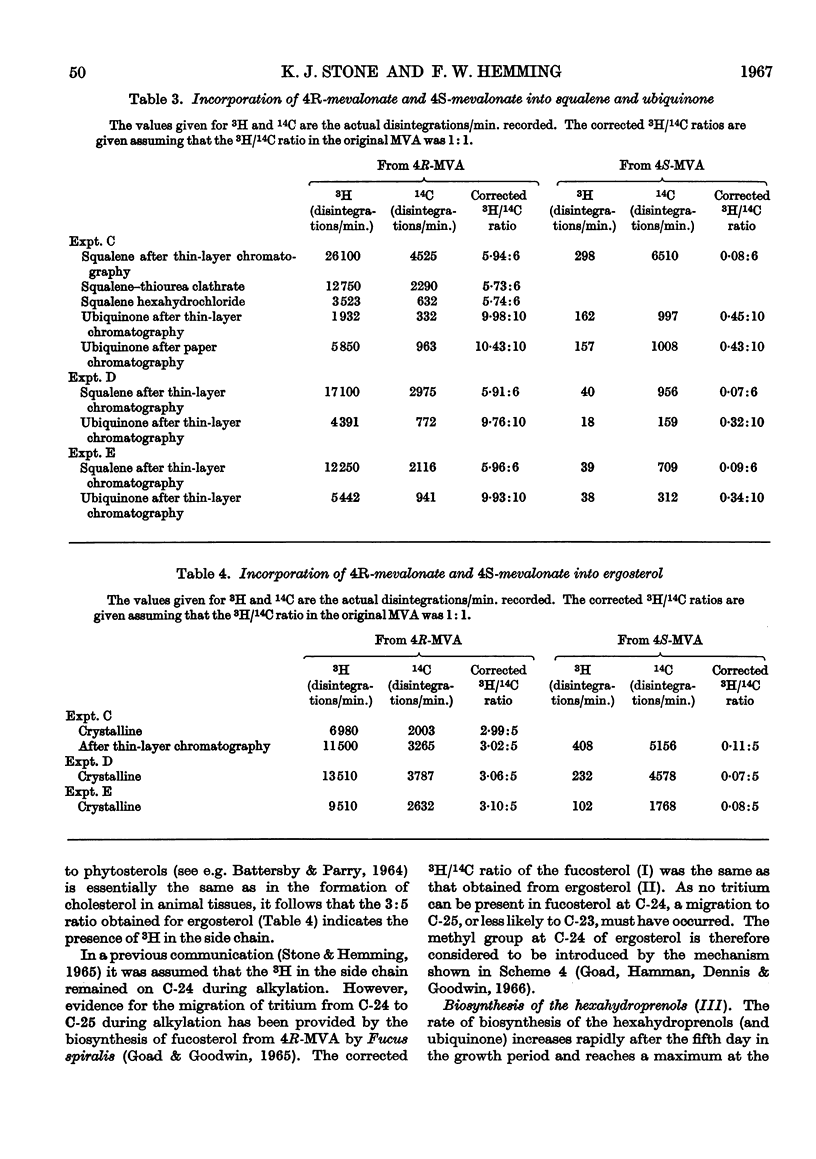


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALEXANDER G. J., GOLD A. M., SCHWENK E. Biogenesis of yeast sterols. III. The origin of carbon 28 of ergosterol. J Biol Chem. 1958 Jun;232(2):599–609. [PubMed] [Google Scholar]
- Anslow W. K., Raistrick H. Studies in the biochemistry of micro-organisms: Fumigatin (3-hydroxy-4-methoxy-2:5-toluquinone), and spinulosin (3:6-dihydroxy-4-methoxy-2:5-toluquinone), metabolic products respectively of Aspergillus fumigatus Fresenius and Penicillium spinulosum Thom. Biochem J. 1938 Apr;32(4):687–696. doi: 10.1042/bj0320687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENNETT R. D., HEFTMANN E. DEVICES FOR CONTINUOUS DEVELOPMENT AND SAMPLE APPLICATION IN PREPARATIVE THIN-LAYER CHROMATOGRAPHY. J Chromatogr. 1963 Oct;12:245–248. doi: 10.1016/s0021-9673(01)83677-3. [DOI] [PubMed] [Google Scholar]
- Cornforth J. W., Cornforth R. H., Donninger C., Popják G. Studies on the biosynthesis of cholesterol XIX. Steric course of hydrogen eliminations and of C-C bond formations in squalene biosynthesis. Proc R Soc Lond B Biol Sci. 1966 Jan 18;163(993):492–514. doi: 10.1098/rspb.1966.0004. [DOI] [PubMed] [Google Scholar]
- Dunphy P. J., Kerr J. D., Pennock J. F., Whittle K. J., Feeney J. The plurality of long chain isoprenoid alcohols (polyprenols) from natural sources. Biochim Biophys Acta. 1967 Feb 7;136(1):136–147. doi: 10.1016/0304-4165(67)90329-7. [DOI] [PubMed] [Google Scholar]
- Dunphy P. J., Whittle K. J., Pennock J. F. On the use of fluorescein and dichlorofluorescein as non-destructive stains for lipids. Chem Ind. 1965 Jul 3;27:1217–1218. [PubMed] [Google Scholar]
- Goad L. J., Hammam A. S., Dennis A., Goodwin T. W. Biosynthesis of the phytosterol side chain. Nature. 1966 Jun 25;210(5043):1322–1324. doi: 10.1038/2101322a0. [DOI] [PubMed] [Google Scholar]
- LAVATE W. V., BENTLEY R. DISTRIBUTION OF NORMAL ISOPRENOLOGS OF COENZYME Q AND DIHYDRO COENZYME Q10 IN VARIOUS MOLDS. Arch Biochem Biophys. 1964 Nov;108:287–291. doi: 10.1016/0003-9861(64)90389-3. [DOI] [PubMed] [Google Scholar]
- Pennock J. F., Hemming F. W., Kerr J. D. A reassessment of tocopherol in chemistry. Biochem Biophys Res Commun. 1964 Nov 30;17(5):542–548. doi: 10.1016/0006-291x(64)90062-2. [DOI] [PubMed] [Google Scholar]
- Rees H. H., Mercer E. I., Goodwin T. W. The stereospecific biosynthesis of plant sterols and alpha- and beta-amyrin. Biochem J. 1966 Jun;99(3):726–734. doi: 10.1042/bj0990726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone K. J., Butterworth P. H., Hemming F. W. Characterization of the hexahydropolyprenols of Aspergillus fumigatus Fresenius. Biochem J. 1967 Feb;102(2):443–455. doi: 10.1042/bj1020443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone K. J., Wellburn A. R., Hemming F. W., Pennock J. F. The characterization of ficaprenol-10, -11 and 12 from the leaves of Ficus elastica (decorative rubber plant). Biochem J. 1967 Jan;102(1):325–330. doi: 10.1042/bj1020325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellburn A. R., Stevenson J., Hemming F. W., Morton R. A. The characterization and properties of castaprenol-11, -12 and -13 from the leaves of Aesculus hippocastanum (horse chestnut). Biochem J. 1967 Jan;102(1):313–324. doi: 10.1042/bj1020313. [DOI] [PMC free article] [PubMed] [Google Scholar]


