Abstract
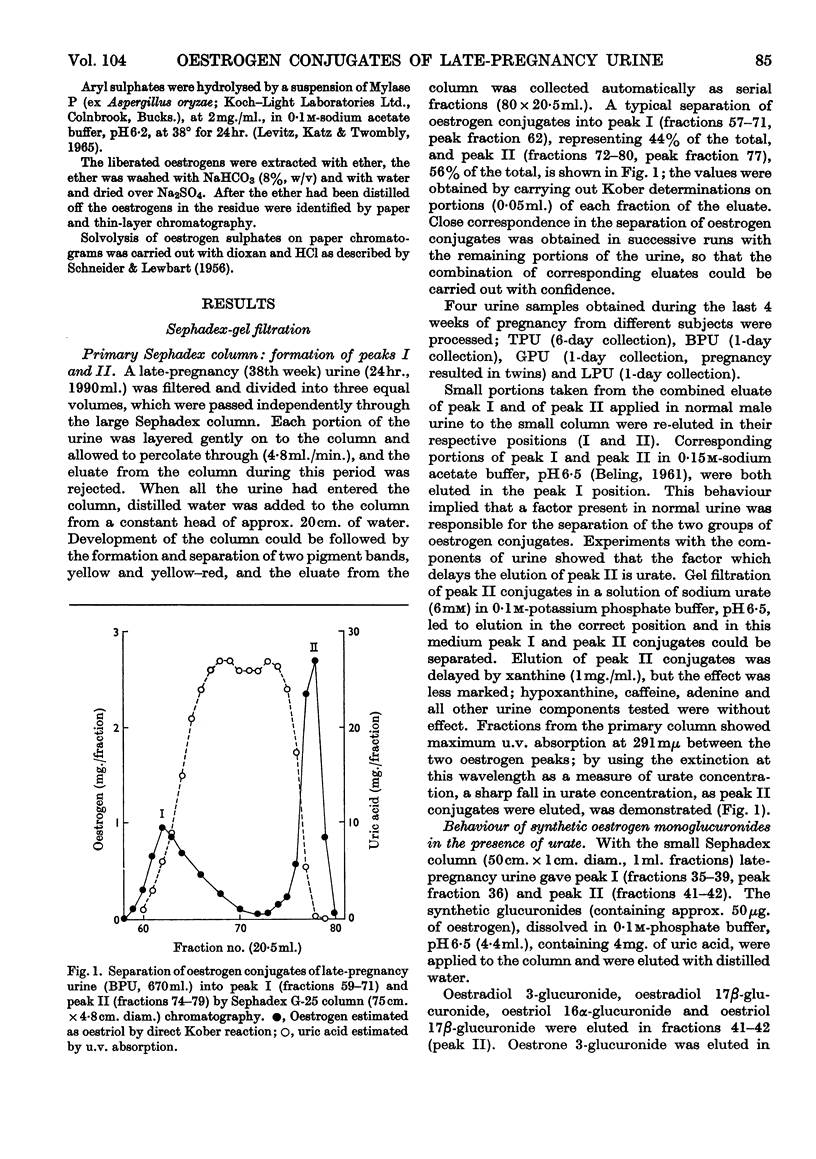
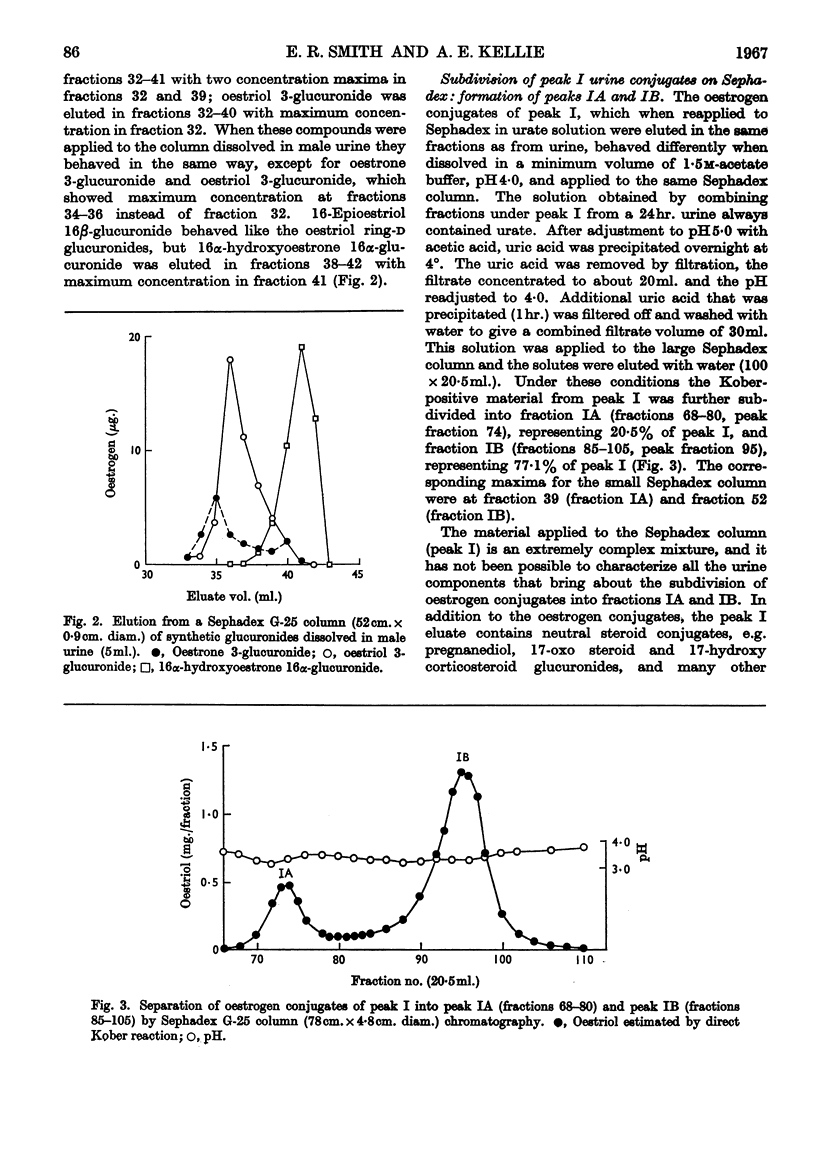
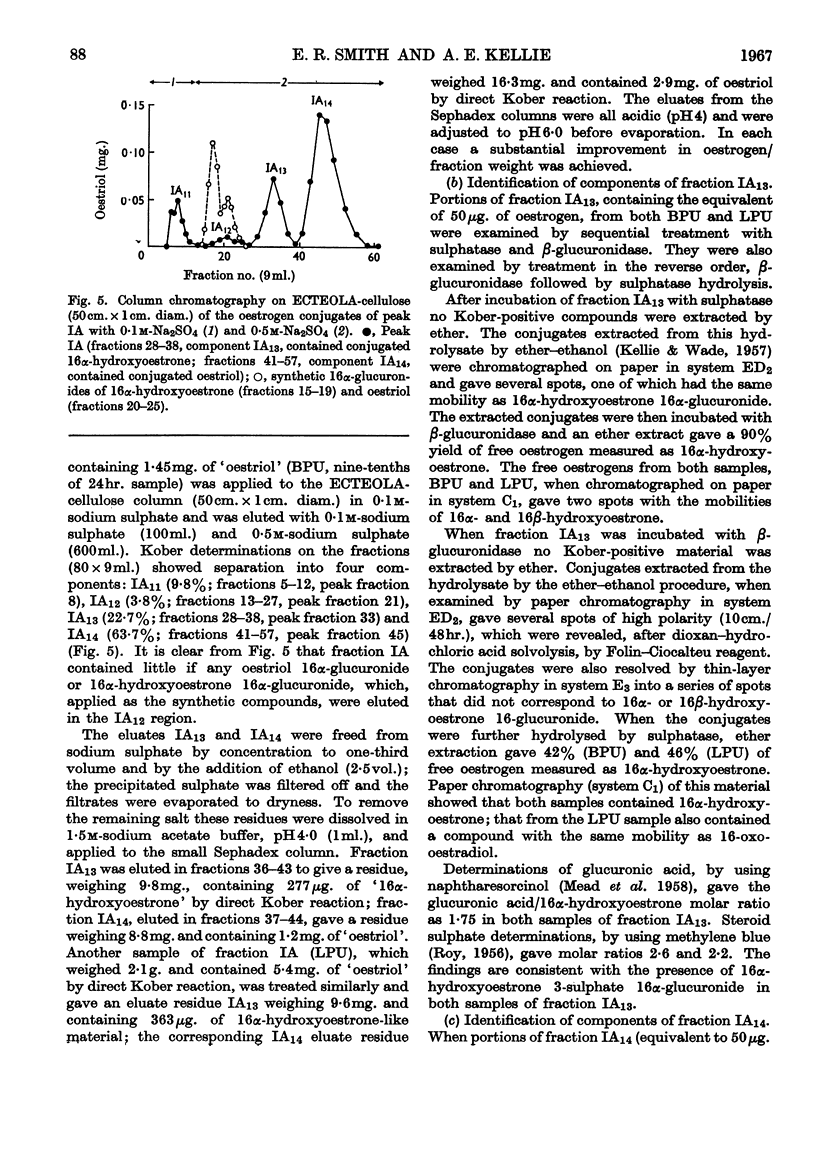
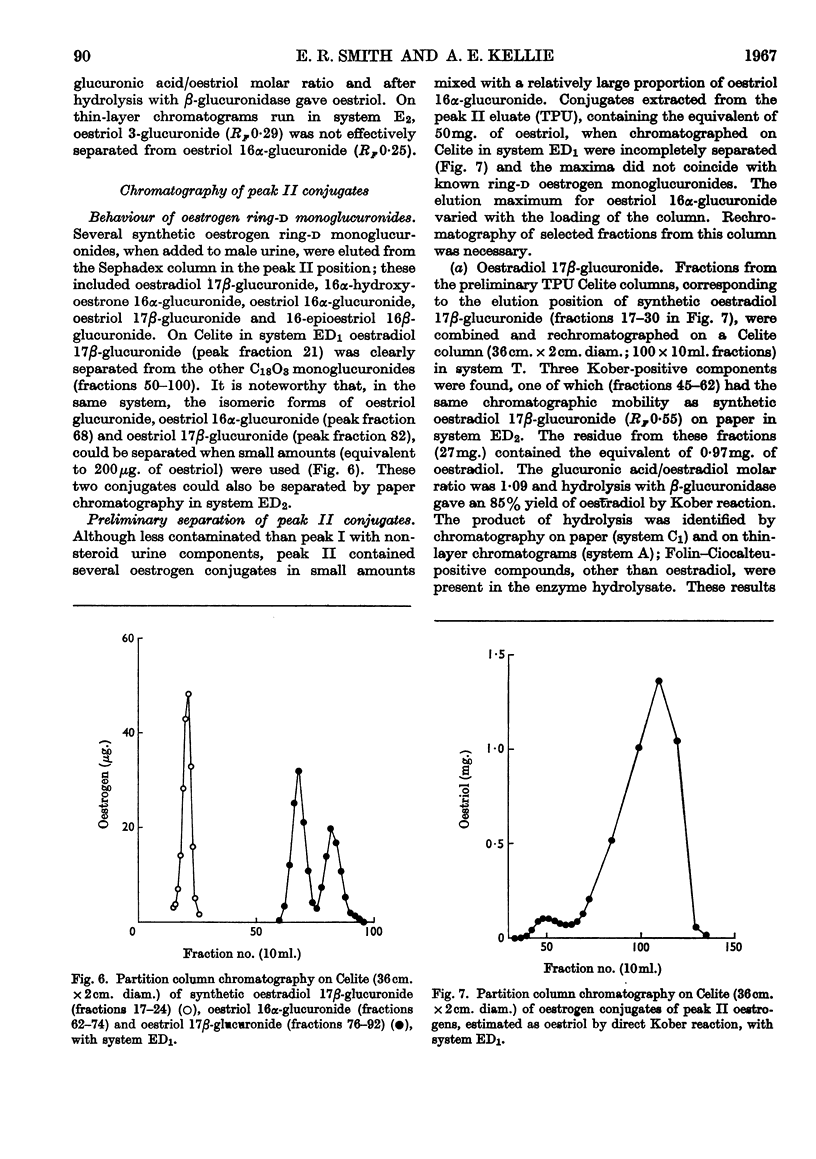
1. The separation of the oestrogen conjugates in late-pregnancy urine into two groups, peaks I and II, by gel filtration on Sephadex G-25 (Beling, 1963) has been shown to be affected by the presence of urate, which delays the elution of peak II conjugates. 2. By reapplication to a Sephadex column, peak I conjugates have been further separated into two groups (peaks IA and IB) and the metabolites in urine effecting this separation have been studied. 3. Further analysis of the mixed conjugates in the main groups IA, IB and II by ion-exchange and partition chromatography has led to the identification of some of the conjugates present. 4. Oestriol 3-sulphate 16α-glucuronide and 16α-hydroxyoestrone 3-sulphate 16α-glucuronide have been identified in peak IA. 5. The main components of peak IB have been identified as oestrone 3-glucuronide and oestriol 3-glucuronide. 6. The major conjugate in peak II was oestriol 16α-glucuronide and no oestriol 17β-glucuronide was found; small amounts of the ring-d monoglucuronides oestradiol 17β-glucuronide, 16-epioestriol 16β-glucuronide and 16α-hydroxyoestrone 16α-glucuronide were found in this fraction. 7. The behaviour of synthetic oestrogen monoglucuronides has been used as a guide in separation.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BELING C. G. Purification of urinary conjugated oestrogens by gel filtration. Nature. 1961 Oct 28;192:326–327. doi: 10.1038/192326a0. [DOI] [PubMed] [Google Scholar]
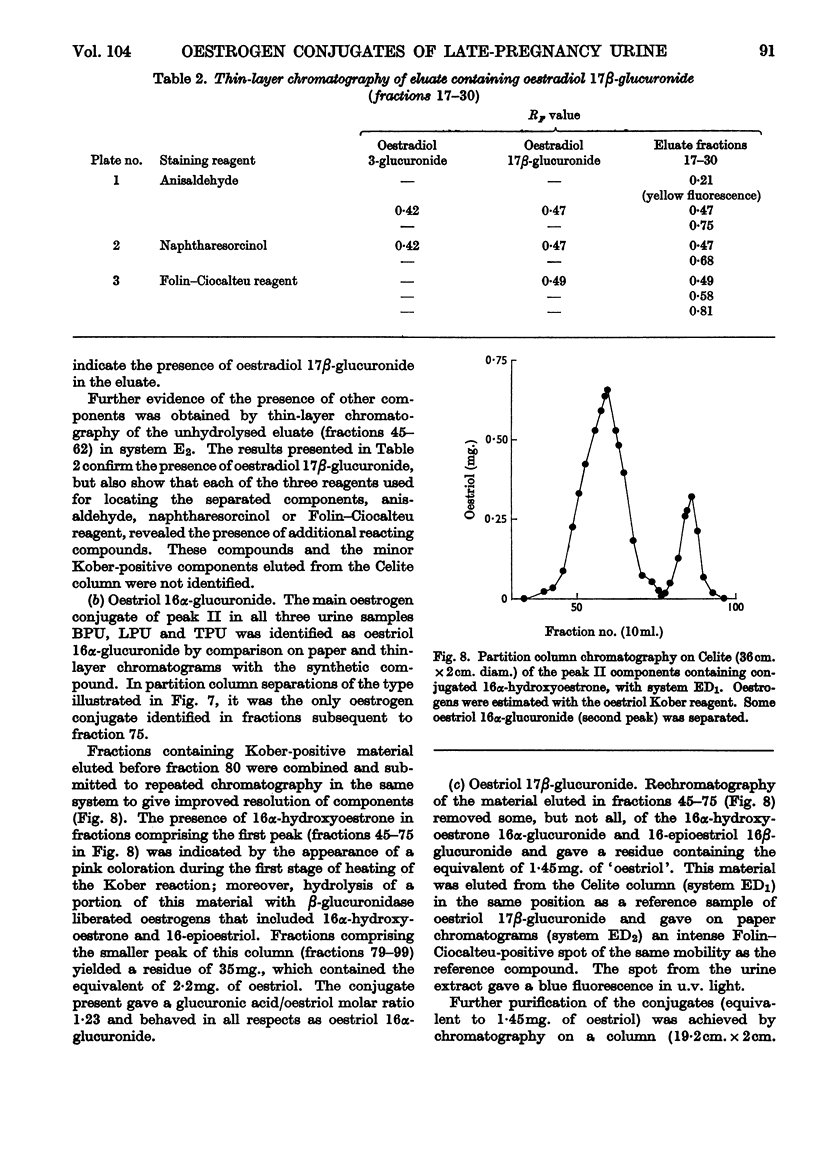
- CARPENTER J. G., KELLIE A. E. The structure of urinary oestriol monoglucuronide. Biochem J. 1962 Aug;84:303–307. doi: 10.1042/bj0840303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. L., Marrian G. F., Odell A. D. Oestriolglycuronide. Biochem J. 1936 Dec;30(12):2250–2256. doi: 10.1042/bj0302250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. L., Marrian G. F. The isolation and identification of a combined form of oestriol in human pregnancy urine. Biochem J. 1936 Jan;30(1):57–65. doi: 10.1042/bj0300057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DICZFALUSY E., BARR M., LIND J. OESTRIOL METABOLISM IN AN ANENCEPHALIC MONSTER. Acta Endocrinol (Copenh) 1964 Aug;46:511–524. doi: 10.1530/acta.0.0460511. [DOI] [PubMed] [Google Scholar]
- DODGSON K. S., SPENCER B. Studies on sulphatases. 4. Arylsulphatase and beta-glucuronidase concentrates from limpets. Biochem J. 1953 Sep;55(2):315–320. doi: 10.1042/bj0550315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elce J. S., Carpenter J. G., Kellie A. E. The synthesis of oestrogen monoglucuronides. J Chem Soc Perkin 1. 1967;7:542–550. doi: 10.1039/j39670000542. [DOI] [PubMed] [Google Scholar]
- GRANT J. K., MARRIAN G. F. Identification of the uronic acid from oestriol 'monoglucuronide.'. Biochem J. 1950 Jun-Jul;47(1):1–3. doi: 10.1042/bj0470001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebelsmann U., Sjöberg K., Wiqvist N., Diczfalusy E. Oestriol-3-glucosiduronate, a major urinary metabolite of oestriol and oestriol-16(17?)-glucosiduronate. Acta Endocrinol (Copenh) 1965 Oct;50(2):261–272. doi: 10.1530/acta.0.0500261. [DOI] [PubMed] [Google Scholar]
- HASHIMOTO Y., NEEMAN M. Isolation and characterization of estriol 16 alpha-glucosiduronic acid from human pregnancy urine. J Biol Chem. 1963 Apr;238:1273–1282. [PubMed] [Google Scholar]
- KELLIE A. E., WADE A. P. The analysis of urinary 17-oxo steroids by gradient elution. Biochem J. 1957 Jun;66(2):196–206. doi: 10.1042/bj0660196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEWBART M. L., SCHNEIDER J. J. Enzymatic synthesis of steroid sulfates. J Biol Chem. 1956 Oct;222(2):787–794. [PubMed] [Google Scholar]
- Levitz M., Katz J., Twombly G. H. The biosynthesis of labeled estriol-3-sulfate-16-glucosiduronate. Steroids. 1965 Nov;6(5):553–559. doi: 10.1016/0039-128x(65)90018-8. [DOI] [PubMed] [Google Scholar]
- MITCHELL F. L. Chromatographic isolation and estimation of the natural oestrogens from tissue. Nature. 1952 Oct 11;170(4328):621–622. doi: 10.1038/170621b0. [DOI] [PubMed] [Google Scholar]
- NOCKE W. A study of the colorimetric estimation of oestradiol-17 beta, oestradiol-17 alpha, oestrone, oestriol and 16-epioestriol by the Kober reaction. Biochem J. 1961 Mar;78:593–602. doi: 10.1042/bj0780593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROY A. B. The steroid sulphatase of Patella vulgata. Biochem J. 1956 Jan;62(1):41–50. doi: 10.1042/bj0620041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOUCHSTONE J. C., GREENE J. W., Jr, MCELROY R. C., MURAWEC T. BLOOD ESTRIOL CONJUGATION DURING HUMAN PREGNANCY. Biochemistry. 1963 Jul-Aug;2:653–657. doi: 10.1021/bi00904a006. [DOI] [PubMed] [Google Scholar]
- WILSON R., ERIKSSON G., DICZFALUSY E. OESTRIOL METABOLISM IN PREGNANT WOMEN. Acta Endocrinol (Copenh) 1964 Aug;46:525–543. doi: 10.1530/acta.0.0460525. [DOI] [PubMed] [Google Scholar]


