Abstract
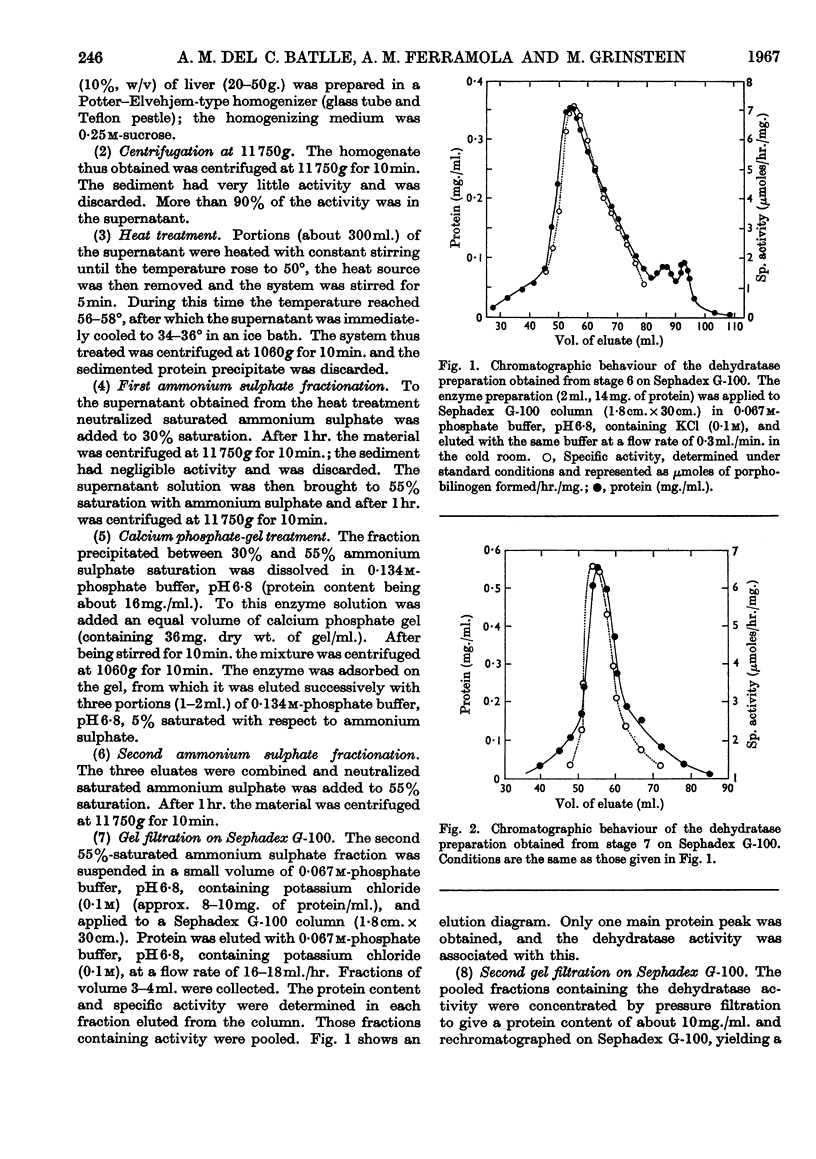
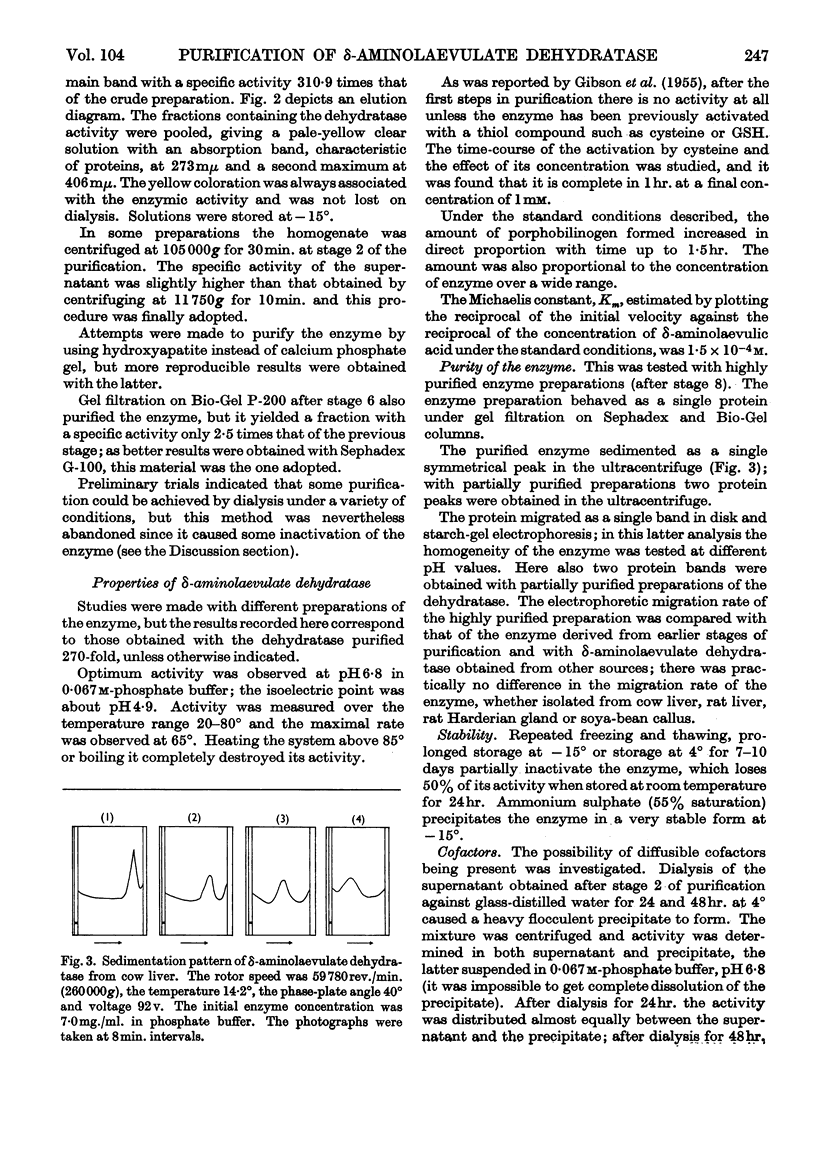
1. δ-Aminolaevulate dehydratase, the enzyme catalysing the condensation of δ-aminolaevulic acid to porphobilinogen, has been prepared from cow liver and its properties have been studied. The enzyme has been purified 310-fold. 2. The purified preparation behaves as a single protein under gel filtration on Sephadex and Bio-Gel columns; it migrates as a single band in disk and starch-gel electro-phoresis at different pH values and it sediments as a single symmetrical peak in the ultracentrifuge. 3. The pH optimum for the pure enzyme was 6·8, the Km at pH 6·8 and 38° was 1·5×10-4m, the isoelectric point was about pH 4·9 and the molecular weight was 140000±14000 by the gel-filtration method. Maximal enzyme activity was observed at 65°. 4. The presence of thiol groups in the enzyme system, essential for its activity, was indicated and the total number of thiol groups was determined. 5. After the first steps of purification the enzyme required cysteine or reduced glutathione for activity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNHAM B. F., PIERCE W. S., WILLIAMS K. R., BOYER M. H., KIRBY C. K. delta-aminolaevulate dehydratase from Rhodopseudomonas spheroides. Biochem J. 1963 Jun;87:462–472. doi: 10.1042/bj0870462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOKSON G. H., RIMINGTON C. Porphobilinogen. Biochem J. 1954 Jul;57(3):476–484. doi: 10.1042/bj0570476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLMAN G. L. A colorimetric method for determining low concentrations of mercaptans. Arch Biochem Biophys. 1958 Apr;74(2):443–450. doi: 10.1016/0003-9861(58)90014-6. [DOI] [PubMed] [Google Scholar]
- GAJDOS A., GAJDOS-TOROK M. Action de certains inhibiteurs et activateurs sur l'acide delta-amino-lévulinique-déshydrogénase. C R Seances Soc Biol Fil. 1955 Dec;149(23-24):2138–2140. [PubMed] [Google Scholar]
- GAJDOS A., GAJDOS-TOROK M. Le métabolisme du porphobilinogène. I. Lieu de formation du porphobilinogène; action de certains effecteurs. Rev Fr Etud Clin Biol. 1956 Nov;1(9):966–972. [PubMed] [Google Scholar]
- GIBSON K. D., NEUBERGER A., SCOTT J. J. The purification and properties of delta-aminolaevulic acid dehydrase. Biochem J. 1955 Dec;61(4):618–629. doi: 10.1042/bj0610618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANICK S. Enzymatic conversion of delta-amino levulinic acid to porphobilinogen. Science. 1954 Dec 31;120(3131):1105–1106. doi: 10.1126/science.120.3131.1105. [DOI] [PubMed] [Google Scholar]
- GRANICK S., MAUZERALL D. Pbrphyrin biosynthesis in erythrocytes. II. Enzymes converting gamma-aminolevulinic acid to coproporphyrinogen. J Biol Chem. 1958 Jun;232(2):1119–1140. [PubMed] [Google Scholar]
- LASCELLES J. Adaptation to form bacteriochlorophyll in Rhodopseudomonas spheroides: changes in activity of enzymes concerned in pyrrole synthesis. Biochem J. 1959 Jul;72:508–518. doi: 10.1042/bj0720508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LASCELLES J. The synthesis of enzymes concerned in bacteriochlorophyll formation in growing cultures of Rhodopseudomonas spheroides. J Gen Microbiol. 1960 Dec;23:487–498. doi: 10.1099/00221287-23-3-487. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAUZERALL D., GRANICK S. The occurrence and determination of delta-amino-levulinic acid and porphobilinogen in urine. J Biol Chem. 1956 Mar;219(1):435–446. [PubMed] [Google Scholar]
- MOORE D. J., LABBE R. F. A QUANTITATIVE ASSAY FOR URINARY PORPHOBILINOGEN. Clin Chem. 1964 Dec;10:1105–1111. [PubMed] [Google Scholar]
- NEUBERGER A., SCOTT J. J. Aminolaevulinic acid and porphyrin biosynthesis. Nature. 1953 Dec 12;172(4389):1093–1094. doi: 10.1038/1721093a0. [DOI] [PubMed] [Google Scholar]
- WALERYCH W. 5-Aminolevulinic acid dehydratase from propionic acid bacteria. Acta Biochim Pol. 1963;10:243–252. [PubMed] [Google Scholar]
- WILDY J., NIZET A., BENSON A. Identification of a plasma material stimulating haemoglobin synthesis in vitro. Biochim Biophys Acta. 1961 Dec 23;54:414–423. doi: 10.1016/0006-3002(61)90080-4. [DOI] [PubMed] [Google Scholar]
- del Batlle A. M., Benson A., Rimington C. Purification and properties of coproporphyrinogenase. Biochem J. 1965 Dec;97(3):731–740. doi: 10.1042/bj0970731. [DOI] [PMC free article] [PubMed] [Google Scholar]



