Abstract
With Streptococcus pneumoniae, moxifloxacin was 4- and 10-fold more effective than levofloxacin at restricting selection of resistant mutants and at killing resistant mutants, respectively. The selection frequency for first-step topoisomerase mutants was 1,000 times lower for moxifloxacin than for levofloxacin; this difference was lost when second-step mutants were selected.
Resistance to penicillin and macrolides is widespread among isolates of Streptococcus pneumoniae (6), and use of fluoroquinolones for treatment of pneumonia has increased (3). Decreased fluoroquinolone susceptibility is being reported (3), and resistant isolates have been recovered from patients after levofloxacin has failed to effect a cure (15, 16, 19; R. J. Davidson, J. DeAzavedo, D. Bast, J. Arbique, R. Bethune, C. Duncan, A. McGeer, and D. E. Low, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 2103, p.127, 2000; N. O. Fishman, B. Suh, L. M. Weigel, B. Lorber, S. Gelone, A. L. Truant, and T. D. Gootz, 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 825, p. 111, 1999). While the prevalence of resistance to levofloxacin is still low in the United States, a five- to sixfold increase in resistance was recorded between surveys of 1997 to 1998 and 1998 to 1999 (18). With several bacteria, addition of a C-8-methoxy group to N-1 cyclopropyl fluoroquinolones improves potency against gyrase and topoisomerase IV resistance mutants (7, 12-14, 20-22), and work with clinical isolates of S. pneumoniae suggests that the C-8-methoxy compound moxifloxacin might restrict the selection of resistant mutants more effectively than levofloxacin (1). The present study compared moxifloxacin and levofloxacin for selection of resistant mutants of S. pneumoniae in vitro.
S. pneumoniae strain ATCC 49619 was grown as liquid cultures in Todd-Hewitt broth (THB; Difco, Detroit, Mich.) containing 10% sheep blood (Hemostat, Dixon, Calif.). For selection of resistant mutants, late-exponential-stage cultures were applied to brain heart infusion (BHI; Difco) agar plates (≤109 CFU/150-mm-diameter plate) containing 10% sheep blood and either moxifloxacin (Bayer Corporation, West Haven, Conn.) or levofloxacin (R. W. Johnson Pharmaceutical Research Institute, Spring House, Pa.). Colonies were recovered and retested for growth on the selecting concentration of fluoroquinolone. DNA was isolated, quinolone-resistance-determining regions (QRDRs) were amplified, and nucleotide sequences were determined as described previously (1, 19). Lethal activity was determined by incubating bacteria grown as liquid cultures (about 3 × 107 CFU/ml) with fluoroquinolone at 37°C for 16 h. Aliquots from samples diluted into cold THB were spotted in triplicate onto drug-free BHI agar containing 10% sheep blood. Colonies were counted after overnight incubation at 37°C in the presence of 5% CO2.
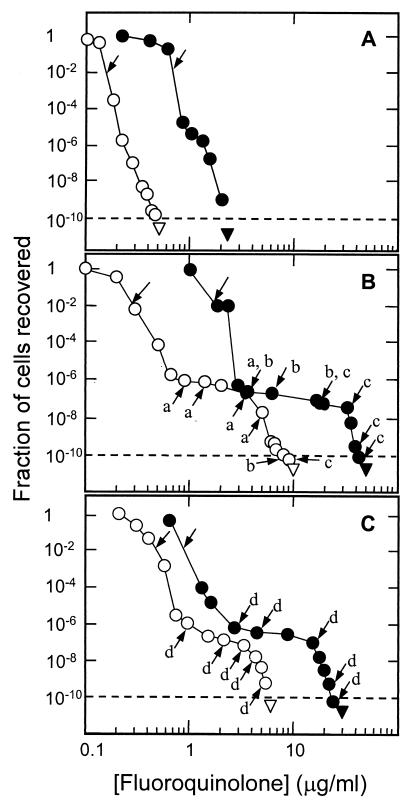
Fluoroquinolone concentration dramatically affected the recovery of resistant mutants. As the concentration increased, the fraction of input cells recovered as mutant colonies dropped sharply, passed through an inflection point, and then continued to drop sharply (Fig. 1A).When the QRDRs of genes encoding DNA gyrase (gyrA and gyrB) and DNA topoisomerase IV (parC and parE) were determined, GyrA variants (Ser-81 to Tyr and Ser-81 to Phe) were detected following moxifloxacin challenge, but only when 109 or more cells were tested. No mutation was found in gyrB, parC, or parE. With levofloxacin, ParC variants (Ser-79 to Tyr and Asp-83 to His) were recovered, but only when at least 1.8 × 106 cells were tested. No mutation was detected in gyrA, gyrB, or parE. These data confirm that DNA gyrase is the primary target for moxifloxacin, while topoisomerase IV is the primary target for levofloxacin (15, 16). The 1,000-fold difference in selection of target mutants has been observed with other pairs of fluoroquinolones (9). It may reflect intrinsic differences between the targets. (With Escherichia coli, parC resistance mutations are codominant with the wild type, while gyrA mutations are recessive [10, 11].) Non-topoisomerase mutants were recovered at low concentration: for some, reserpine lowered the fluoroquinolone MIC (not shown), indicating involvement of active efflux in reduced fluoroquinolone susceptibility (2). The concentration at which no mutant was recovered when more than 1010 cells were tested (mutant prevention concentration [MPC]) (8) was fourfold lower for moxifloxacin (Fig. 1A, arrowheads, and Table 1).
FIG. 1.
Effect of fluoroquinolone concentration on the recovery of single-step, resistant mutants. S. pneumonia was grown to late log phase and then distributed at various dilutions to agar plates containing the indicated concentrations of moxifloxacin (open circles) or levofloxacin (solid circles). After incubation, colonies were counted, and the fraction recovered was determined relative to the number of input CFU. Unlabeled arrows indicate MIC99. The triangle indicates no colony recovered at that drug concentration and bacterial load. The dashed line indicates one colony recovered per 1010 cells tested. Similar results were obtained in two independent experiments. Three strains were tested: wild-type strain ATCC 49619 (A); strain KD2138, a ParC (Ser-79 to Tyr) variant (B); and strain KD2139, a GyrA (Ser-81 to Phe) variant (C). Two double mutants were recovered from each point indicated by an arrow, and the nucleotide sequence of the QRDRs of gyrA and parC was determined for each mutant. Letters indicate recovery of the following mutants: a (GyrA Ser-81 to Phe), b (GyrA Ser-81 to Tyr), c (GyrA Glu-85 to Lys), and d (Par C Ser-79 to Tyr). A single letter with an arrow indicates that both mutants examined had the same change.
TABLE 1.
Effect of gyrA and parC resistance mutations on fluoroquinolone activity
| S. pneumoniae strain | MIC99 (μg/ml)a
|
MPC (μg/ml)a
|
LD99 (μg/ml)b
|
% Survival at wild-type MPCb
|
||||
|---|---|---|---|---|---|---|---|---|
| Moxi- floxacin | Levo- floxacin | Moxi- floxacin | Levo- floxacin | Moxi- floxacin | Levo- floxacin | Moxi- floxacin | Levo- floxacin | |
| ATCC 49619 (wild type) | 0.15 | 0.7 | 0.5 | 2.3 | 0.22 | 1.2 | 0.004 | 0.008 |
| KD2139 (gyrAr) | 0.56 | 0.9 | 6 | 30 | 0.6 | 7.5 | 2.2 | 80c |
| KD2138 (parCr) | 0.29 | 1.7 | 10 | 50 | 0.6 | 6.7 | 1.7 | 170c |
Data obtained from Fig. 1. A replicate experiment in each case gave similar values.
Data obtained from Fig. 2. A replicate experiment in each case gave similar values.
Survival was determined relative to that of cells present immediately before addition of fluoroquinolone. Survival >100% indicates cell growth.
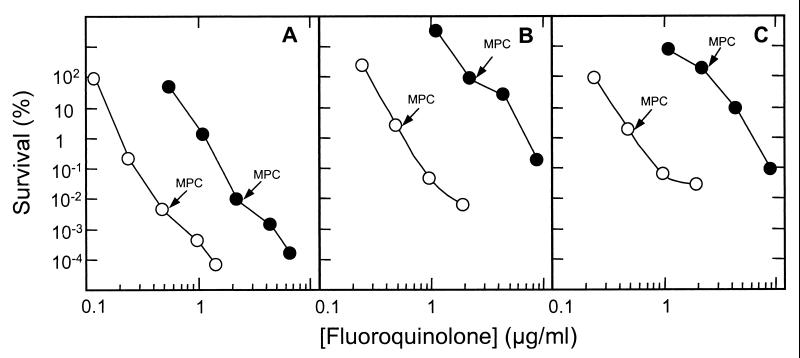
When second-step mutants were selected from a first-step parC resistance strain (KD2138) by using moxifloxacin or levofloxacin, the inflection region was more pronounced than with wild-type cells, and the MPC was higher (Fig. 1B). Determination of QRDR nucleotide sequences in mutant DNA showed that gyrA parC double mutants were recovered (labeled arrows, Fig. 1B). Similar phenomena were observed with a first-step gyrA resistance mutant (strain KD2139; Fig. 1C). For both mutants, the MPC was higher for levofloxacin. This may reflect the inability of levofloxacin to kill resistant mutants at the MPC (Fig. 2 and Table 1).
Several parameters obtained from the experiments described above allow quantitative comparison of moxifloxacin and levofloxacin (Table 1). First, moxifloxacin MICs required to block colony formation by 99% (MIC99; unlabeled arrows in Fig. 1) were 21, 62, and 17% of that of levofloxacin with wild-type, gyrA-resistant, and parC-resistant cells, respectively. The difference between the two mutants reflects the target preference of the two compounds (16, 17). When the MPC is taken as the ability to restrict the growth of the most resistant mutant, moxifloxacin was four to five times more effective with wild-type cells and resistant mutants. With respect to lethal activity, moxifloxacin concentrations that allowed only 1% survival (LD99) were 18, 8, and 9% that of levofloxacin with wild-type, gyrA-resistant, and parC-resistant cells, respectively. Collectively, these data show that moxifloxacin is intrinsically more active against wild-type and resistant S. pneumoniae.
From published pharmacokinetic measurements (4, 5, 18a), we calculated that drug concentrations in serum were above the MPC longer for moxifloxacin (38 h at the 400-mg dose) than for levofloxacin (8 h at the 500-mg dose; 18 h at the 750-mg dose). The area under the time-concentration curve that was above the MPC during 24 h for moxifloxacin was 4.5 times greater than that for levofloxacin (500-mg dose). Collectively, these data support the previous conclusion (1) that drug concentrations in serum are more likely to exceed the MPC of moxifloxacin than that of levofloxacin with S. pneumoniae. Additional work is required to provide estimates of available drug at the relevant sites of infection.
The acquisition of a first-step parC mutation by levofloxacin may have only a small effect on the ability of moxifloxacin to prevent growth (compare MIC99s in Table 1); however, it increases by several orders of magnitude the probability for acquiring a second mutation (compare Fig. 1A with B). Thus, preservation of the effectiveness of new fluoroquinolones, such as moxifloxacin and gemifloxacin, may require avoiding the selective enrichment of first-step mutants by older compounds such as ciprofloxacin and levofloxacin.
FIG. 2.
Survival of S. pneumoniae in the presence of fluoroquinolone. Cells were incubated in the presence of the indicated concentrations of moxifloxacin (open circles) or levofloxacin (solid circles), after which they were plated on drug-free agar. Percent survival was calculated relative to CFU at the time of drug addition. (A) Wild-type strain ATCC 49619. (B) gyrA resistance mutant strain KD2139. (C) parC resistance mutant KD2138. MPCs, determined as described in the legend to Fig. 1, are indicated by arrows. Similar results were obtained in a replicate experiment.
Acknowledgments
We thank Marila Gennaro, Samuel Kayman, and Glenn Tillotson for critical comments on the manuscript.
This work was supported by Bayer Corp. and NIH grant AI35257.
REFERENCES
- 1.Blondeau, J. M., X. Zhao, G. Hansen, and K. Drlica. 2001. Mutant prevention concentrations of fluoroquinolones for clinical isolates of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 45:433-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brenwald, N. P., M. J. Gill, and R. Wise. 1998. Prevalence of a putative efflux mechanism among fluoroquinolone-resistant clinical isolates of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 42:2032-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen, D. K., A. McGeer, J. C. DeAzavedo, and D. E. Low. 1999. Decreased susceptibility of Streptococcus pneumoniae to fluoroquinolones in Canada. N. Engl. J. Med. 341:233-239. [DOI] [PubMed] [Google Scholar]
- 4.Chien, S.-C., F. A. Wong, C. L. Fowler, S. V. Callery-D'Amico, R. R. Williams, R. Nayak, and A. T. Chow. 1998. Double-blind evaluation of the safety and pharmacokinetics of multiple oral once-daily 750-milligram and 1-gram doses of levofloxacin in healthy volunteers. Antimicrob. Agents Chemother. 42:885-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chien, S.-H., M. C. Rogge, L. G. Gisclon, C. Curtin, F. Wong, J. Natarajan, R. R. Williams, C. L. Fowler, W. K. Cheung, and A. T. Chow. 1997. Pharmacokinetic profile of levofloxacin following once-daily 500-milligram oral or intravenous doses. Antimicrob. Agents Chemother. 41:2256-2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doern, G. 2001. Antimicrobial use and the emergence of antimicrobial resistance with Streptococcus pneumoniae in the United States. Clin. Infect. Dis. 33(Suppl. 3):S187-S192. [DOI] [PubMed] [Google Scholar]
- 7.Dong, Y., C. Xu, X. Zhao, J. Domagala, and K. Drlica. 1998. Fluoroquinolone action against mycobacteria: effects of C-8 substituents on bacterial growth, survival, and resistance. Antimicrob. Agents Chemother. 42:2978-2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong, Y., X. Zhao, J. Domagala, and K. Drlica. 1999. Effect of fluoroquinolone concentration on selection of resistant mutants of Mycobacterium bovis BCG and Staphylococcus aureus. Antimicrob. Agents Chemother. 43:1756-1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fukuda, H., R. Kishi, M. Takei, and M. Hosaka. 2001. Contributions of the 8-methoxy group of gatifloxacin to resistance selectivity, target preference, and antibacterial activity against Streptococcus pneumoniae. Antimicrob. Agents Chemother. 45:1649-1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hane, M. W., and T. H. Wood. 1969. Escherichia coli K-12 mutants resistant to nalidixic acid: genetic mapping and dominance studies. J. Bacteriol. 99:238-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khodursky, A., and N. Cozzarelli. 1998. The mechanism of inhibition of topoisomerase IV by quinolone antibacterials. J. Biol. Chem. 273:27668-27677. [DOI] [PubMed] [Google Scholar]
- 12.Kitamura, A., K. Hoshino, Y. Kimura, I. Hayakawa, and K. Sato. 1995. Contribution of the C-8 substituent of DU-6859a, a new potent fluoroquinolone, to its activity against DNA gyrase mutants of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 39:1467-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu, T., X. Zhao, and K. Drlica. 1999. Gatifloxacin activity against quinolone-resistant gyrase: allele-specific enhancement of bacteriostatic and bactericidal activity by the C-8-methoxy group. Antimicrob. Agents Chemother. 43:2969-2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu, T., X. Zhao, X. Li, A. Drlica-Wagner, J.-Y. Wang, J. Domagala, and K. Drlica. 2001. Enhancement of fluoroquinolone activity by C-8 halogen and methoxy moieties: action against a gyrase resistance mutant of Mycobacterium smegmatis and a gyrase-topoisomerase IV double mutant of Staphylococcus aureus. Antimicrob. Agents Chemother. 45:2703-2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan, X.-S., J. Ambler, S. Mehtar, and L. M. Fisher. 1996. Involvement of topoisomerase IV and DNA gyrase as ciprofloxacin targets in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 40:2321-2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pestova, E., R. Beyer, N. P. Cianciotto, G. A. Noskin, and L. R. Peterson. 1999. Contribution of topoisomerase IV and DNA gyrase mutations in Streptococcus pneumoniae to resistance to novel fluoroquinolones. Antimicrob. Agents Chemother. 43:2000-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pestova, E., J. Millichap, G. Noskin, and L. Peterson. 2000. Intracellular targets of moxifloxacin: a comparison with other fluoroquinolones. J. Antimicrob. Chemother. 45:583-590. [DOI] [PubMed] [Google Scholar]
- 18.Sahm, D. F., J. A. Karlowsky, L. J. Kelly, I. A. Critchley, M. E. Jones, C. Thornsberry, Y. Mauriz, and J. Kahn. 2001. Need for annual surveillance of antimicrobial resistance in Streptococcus pneumoniae in the United States: 2-year longitudinal analysis. Antimicrob. Agents Chemother. 45:1037-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18a.Sullivan, J. T., M. Woodruff, J. Lettieri, V. Agarwal, G. J. Krol, P. T. Leese, S. Watson, and A. H. Heller. 1999. Pharmacokinetics of a once-daily oral dose of moxifloxacin (Bay 12-8039), a new enantiomerically pure 8-methoxy quinolone. Antimicrob. Agents Chemother. 43:2793-2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Urban, C., N. Rahman, X. Zhao, N. Mariano, S. Segal-Maurer, K. Drlica, and J. Rahal. 2001. Fluoroquinolone-resistant Streptococcus pneumoniae associated with levofloxacin therapy. J. Infect. Dis. 184:794-798. [DOI] [PubMed] [Google Scholar]
- 20.Zhao, B.-Y., R. Pine, J. Domagala, and K. Drlica. 1999. Fluoroquinolone action against clinical isolates of Mycobacterium tuberculosis: effects of a C-8 methoxyl group on survival in liquid media and in human macrophages. Antimicrob. Agents Chemother. 43:661-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao, X., J.-Y. Wang, C. Xu, Y. Dong, J. Zhou, J. Domagala, and K. Drlica. 1998. Killing of Staphylococcus aureus by C-8-methoxy fluoroquinolones. Antimicrob. Agents Chemother. 42:956-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao, X., C. Xu, J. Domagala, and K. Drlica. 1997. DNA topoisomerase targets of the fluoroquinolones: a strategy for avoiding bacterial resistance. Proc. Natl. Acad. Sci. USA 94:13991-13996. [DOI] [PMC free article] [PubMed] [Google Scholar]