Abstract
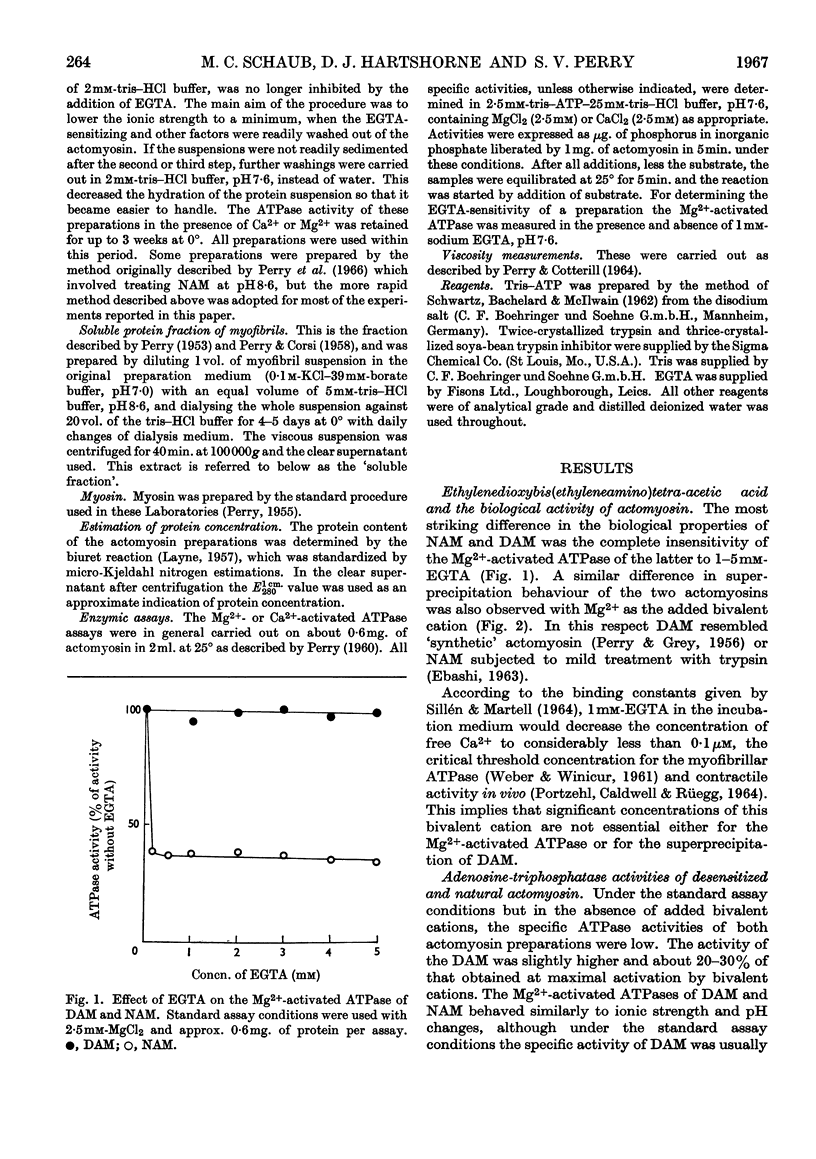
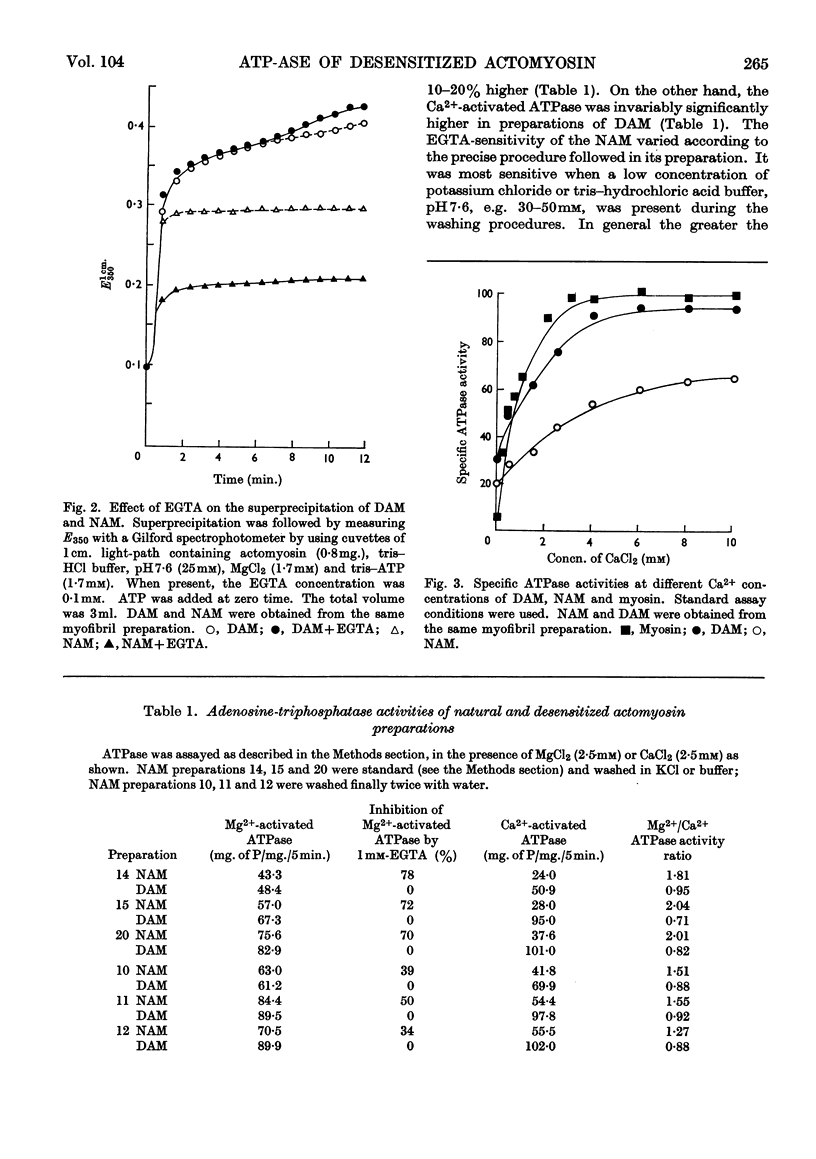
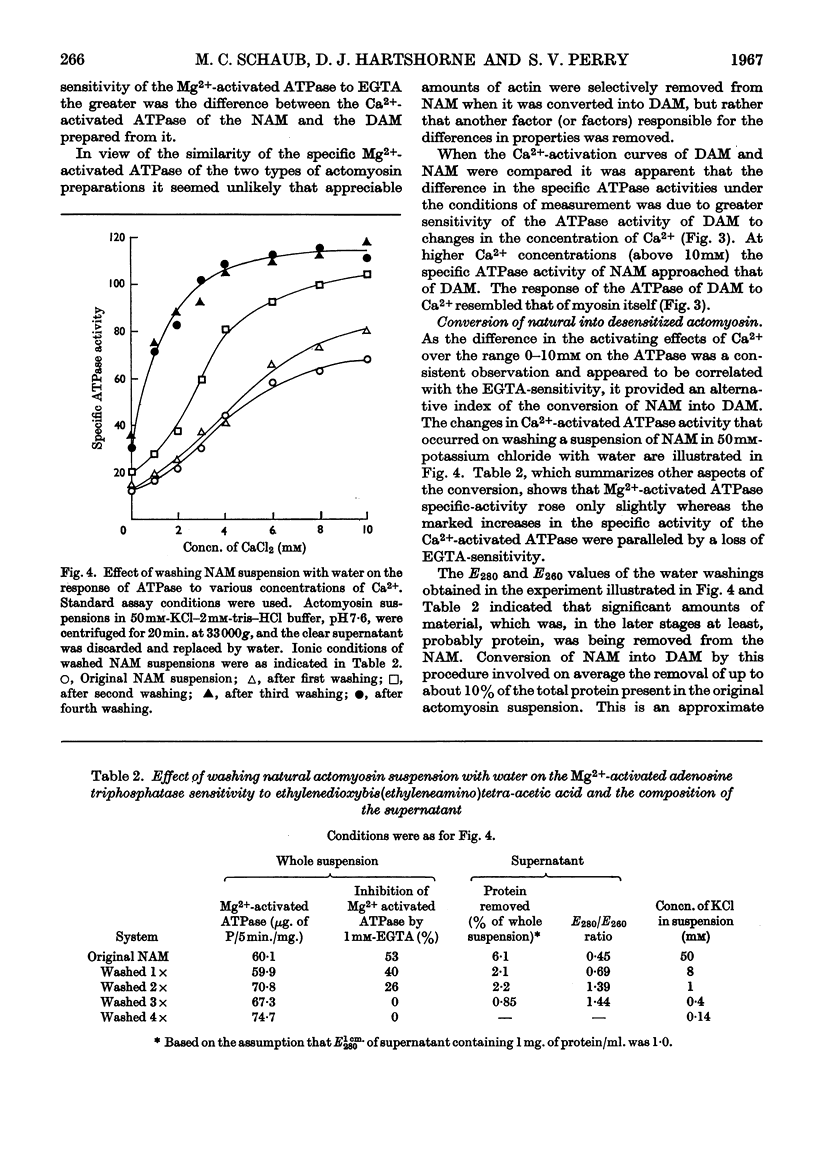
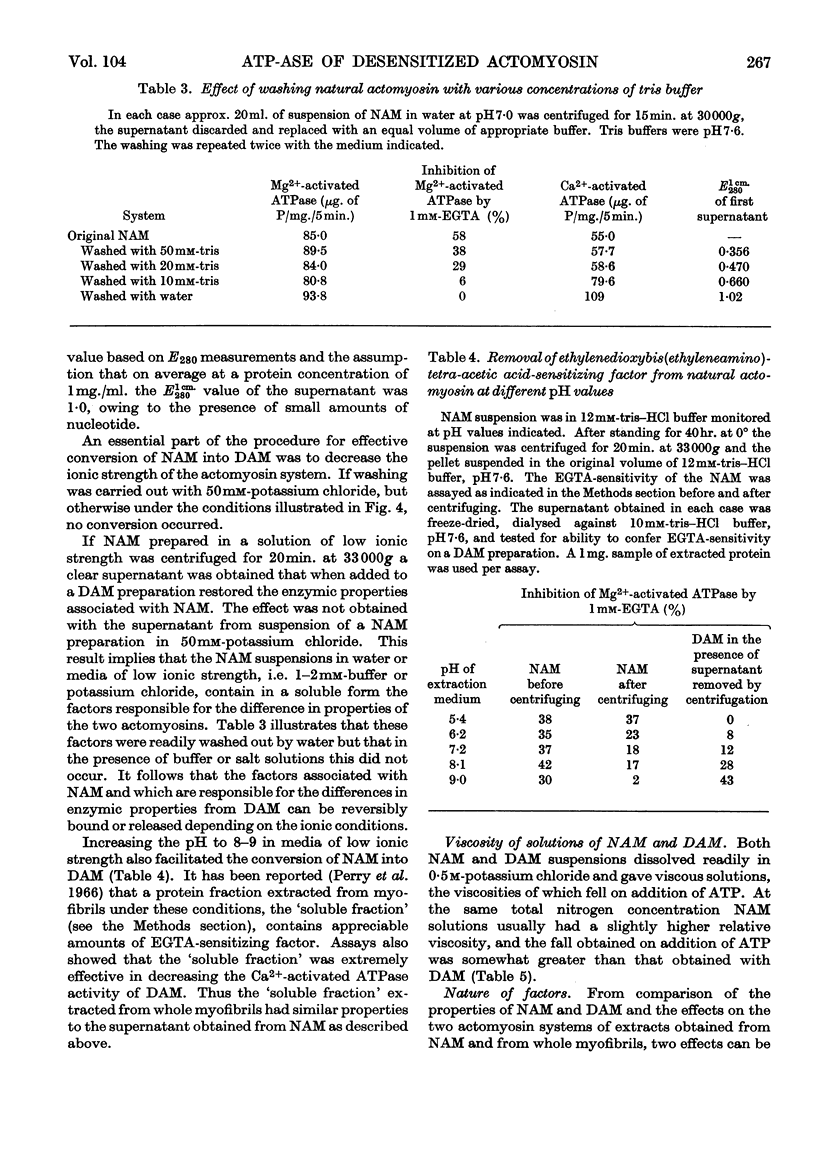
1. A simple procedure involving repeated washings of actomyosin, extracted as the complex from myofibrils (natural actomyosin) at ionic strength less than 0·002, is described for the preparation of a desensitized actomyosin. 2. The Mg2+-activated adenosine triphosphatase of natural actomyosin was markedly inhibited by ethylenedioxybis(ethyleneamino)tetra-acetic acid, whereas that of the desensitized actomyosin was unaffected. 3. The activity of the Ca2+-activated adenosine triphosphatase of natural actomyosin was generally lower than that of the Mg2+-activated adenosine triphosphatase, whereas in the desensitized actomyosin the difference between the activities was considerably less. In both natural and desensitized actomyosin the adenosine-triphosphatase activities in the presence of Mg2+ were similar. 4. The conversion of the natural into the desensitized actomyosin was accompanied by the removal of a protein fraction containing the factors responsible for the sensitivity to ethylenedioxybis(ethyleneamino)tetra-acetic acid and for modifying the Ca2+-activated adenosine triphosphatase. When added to a desensitized actomyosin this fraction effected a reversal to the natural form. The recombination was facilitated by increasing the ionic strength of the medium. The two factors showed different stabilities to heat and tryptic digestion.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAVIES R. E. A MOLECULAR THEORY OF MUSCLE CONTRACTION: CALCIUM-DEPENDENT CONTRACTIONS WITH HYDROGEN BOND FORMATION PLUS ATP-DEPENDENT EXTENSIONS OF PART OF THE MYOSIN-ACTIN CROSS-BRIDGES. Nature. 1963 Sep 14;199:1068–1074. doi: 10.1038/1991068a0. [DOI] [PubMed] [Google Scholar]
- EBASHI S. THIRD COMPONENT PARTICIPATING IN THE SUPERPRECIPITATION OF 'NATURAL ACTOMYOSIN'. Nature. 1963 Dec 7;200:1010–1010. doi: 10.1038/2001010a0. [DOI] [PubMed] [Google Scholar]
- Hartshorne D. J., Perry S. V., Davies V. A factor inhibiting the adenosine triphosphatase activity and the superprecipitation of actomyosin. Nature. 1966 Mar 26;209(5030):1352–1353. doi: 10.1038/2091352a0. [DOI] [PubMed] [Google Scholar]
- KELLY C. D., LAYNE S. Bacteria found in the air over Canada and the American Arctic. Can J Microbiol. 1957 Apr;3(3):447–455. doi: 10.1139/m57-047. [DOI] [PubMed] [Google Scholar]
- PERRY S. V., CORSI A. Extraction of proteins other than myosin from the isolated rabbit myofibril. Biochem J. 1958 Jan;68(1):5–12. doi: 10.1042/bj0680005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRY S. V. The adenosinetriphosphatase activity of myofibrils isolated from skeletal muscle. Biochem J. 1951 Mar;48(3):257–265. doi: 10.1042/bj0480257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRY S. V. The chromatography of L-myosin on diethylaminoethylcellulose. Biochem J. 1960 Jan;74:94–101. doi: 10.1042/bj0740094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRY S. V. The protein components of the isolated myofibril. Biochem J. 1953 Aug;55(1):114–122. doi: 10.1042/bj0550114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRY S. V., ZYDOWO M. The nature of the extra protein fraction from myofibrils of striated muscle. Biochem J. 1959 Feb;71(2):220–228. doi: 10.1042/bj0710220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTZEHL H., CALDWELL P. C., RUEEGG J. C. THE DEPENDENCE OF CONTRACTION AND RELAXATION OF MUSCLE FIBRES FROM THE CRAB MAIA SQUINADO ON THE INTERNAL CONCENTRATION OF FREE CALCIUM IONS. Biochim Biophys Acta. 1964 May 25;79:581–591. doi: 10.1016/0926-6577(64)90224-4. [DOI] [PubMed] [Google Scholar]
- Perry S. V., Cotterill J. The action of thiol inhibitors on the interaction of F-actin and heavy meromyosin. Biochem J. 1964 Sep;92(3):603–608. doi: 10.1042/bj0920603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry S. V., Davies V., Hayter D. Natural tropomyosin and the factor sensitizing actomyosin adenosine triphosphatase to ethylenedioxybis(ethyleneamino)tetra-acetic acid. Biochem J. 1966 Apr;99(1):1C–2C. doi: 10.1042/bj0990001c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWARTZ A., BACHELARD H. S., McIL WAIN H. The sodium-stimulated adenosine-triphosphatase activity and other properties of cerebral microsomal fractions and subfractions. Biochem J. 1962 Sep;84:626–637. doi: 10.1042/bj0840626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBER A., WINICUR S. The role of calcium in the superprecipitation of actomyosin. J Biol Chem. 1961 Dec;236:3198–3202. [PubMed] [Google Scholar]


