Abstract
Abacavir is a potent new carbocyclic nucleoside analogue. We employed our hollow-fiber pharmacodynamic modeling system to examine the antiretroviral effects of different abacavir exposures, as well as the impact of the schedule of drug administration on efficacy. Dose ranging of abacavir revealed that a concentration of four times the 50% effective concentration (EC50) (approximately the EC95) was required to inhibit the replication of human immunodeficiency virus type 1 (HIV-1) (strain MN) either in a continuous-infusion hollow-fiber experiment or in a classical tissue culture flask experiment. In contrast to earlier work with another drug class (HIV-1 protease inhibitors), addition of physiological amounts of the human drug binding proteins albumin and α1 acid glycoprotein revealed that there was little impact on the antiviral effect of the drug. Comparison of equivalent exposures (an area under the concentration-time curve [AUC] developed by approximately 500 mg per day of orally administered abacavir), either in a continuous-infusion mode or as a single oral dose of abacavir, demonstrated no difference in the ability to suppress either strain IIIB or strain MN. Comparison of administration of 250 mg every 12 h (q12h) versus once-daily administration of 500 mg for strain MN again showed no significant difference in suppressive effect. These experiments were carried out over 8 to 15 days. Because of these promising initial results, we extended the experiment to 30 days and examined three different schedules of administration that generated the same AUC at 24 h (AUC24): 300 mg q12h, 600 mg q24h, and 1,200 mg q48h. The aim of the last of these regimens was to definitively demonstrate schedule failure. There was little difference between the 1,200-mg q48h treatment group and the untreated control at 30 days. Likewise, there was little difference between the 600-mg q24h and 300-mg q12h treatment groups. However, at circa day 18 of the experiment, there was a small increase in viral output of p24 in the once-daily dosing unit. Examination of virus from all groups demonstrated no phenotypic or genotypic differences. The small difference in hollow-fiber unit p24 in the once-daily dosing group was not due to emergence of resistance over the 30-day single-drug exposure. We conclude that the dose of abacavir currently being studied in clinical trials (300 mg orally q12h) will be efficacious for the majority of sensitive clinical isolates of HIV-1. These in vitro data also suggest that this drug may be able to be administered to patients on a once-daily basis at a dose of 600 mg.
Abacavir is a new carbocyclic nucleoside analogue with potent anti-human immunodeficiency virus type 1 (HIV-1) activity. In clinical trial work (M. Saag, D. Lancaster, A. Sonnerberg, J. Mulder, R. Torres, R. Schooley, R. Harrigan, D. Kelleher, and W. Symonds, Abstr. Third Conf. Retroviruses Opportunistic Infect., abstr. 195, 1996) administration of abacavir has resulted in the reduction of patients' HIV-1 RNA copy number by 1.5 to 2.0 log10, on the order of that seen with HIV-1 protease inhibitors.
Our group has developed an in vitro pharmacodynamic system that can aid the design of phase I/II studies. We have developed a flowpath that uses computer driven pumps to expose multiple hollow-fiber bioreactors to fluctuating drug concentrations that simulate the human pharmacokinetic profiles of candidate anti-HIV therapeutics. By using mixtures of uninfected and HIV-infected human lymphocytes, the minimum effective dose can be determined. Dose fractionation experiments can then yield information on whether a schedule (e.g., half the daily dose every 12 h [q12h] or one-third the daily dose q8h) will effectively limit HIV-1 replication and spread within the culture. Results to date have indicated good agreement between dose and schedule predicted by this in vitro system and clinical phase I/II trials (1, 2). The hollow-fiber unit has correctly predicted the clinical dose of stavudine and has also correctly predicted that q12h dosing would be as efficacious as more-fractionated schedules (actually continuous infusion of the same daily area under the concentration-time curve [AUC]). Hollow-fiber pharmacodynamic evaluation also correctly predicted the lack of success of the protease inhibitor A-77003, mainly because of protein binding issues. An important issue, however, is that the hollow-fiber system has been of utility for at least two different classes of antiretroviral agents.
MATERIALS AND METHODS
Cell culture.
Human T-lymphoblastoid cell lines CEM, MT-2, and H9 cells infected with the IIIB and MN strains of HIV-1 were originally obtained from the AIDS Research and Reference Reagent Program, AIDS Program, National Institute of Allergy and Infectious Diseases, Bethesda, Md. The cloned HIV-1 strain HXB-2 was obtained from M. Tisdale (Glaxo-Wellcome, Greenford, United Kingdom) and was maintained in CEM cells in standard and hollow-fiber culture. Cells were cultured in RPMI 1640 medium containing 10% (vol/vol) fetal bovine serum, 25 mM HEPES, and gentamicin (50 μg/ml; Paragon Biotech, Baltimore, Md.). Medium for hollow-fiber bioreactor culture was modified for growth in the absence of CO2 by the omission of Na2HCO3 during formulation. Hollow-fiber bioreactors with a 50,000-MW cutoff (Spectrum-Cellco, Los Angeles, Calif.) were used for this study. Experimental hollow-fiber units (previously perfused for 16 to 24 h with test medium) were initiated by introduction of 7 ml of a 1:100 mixture of HIV-1-infected and uninfected CEM cells at a density of 3 × 106 cells per ml into the extracapillary space (ECS). An initial sample was taken for determination of cell number, percentage of infected cells, viability by trypan blue exclusion, p24 level, and DNA PCR. In continuous-infusion experiments the drug-containing medium was recirculated, and replenishment of drug-containing medium was done daily due to adequate stability of the agent.
HIV antigen assay.
We employed p24 determination as the endpoint because previous work (1, 2) has demonstrated equivalence of outcome when employing p24, infectious-center assays, or RNA PCR determination of viral load in this system. Samples for p24 antigen assay were taken at the indicated time points after initiation of experiments. Aliquots of cells and tissue culture media were removed through the ECS access ports on the bioreactors at the indicated time points and centrifuged for 5 min at 1,500 × g. HIV-1 p24 protein in cell culture supernatant was measured using the Coulter p24 enzyme-linked immunosorbent assay according to the manufacturer's guidelines (Beckman Coulter, Palo Alto, Calif.). Absorbance was measured and data analyzed using a computer supported microplate reader (Molecular Devices, Menlo Park, Calif.). Levels of the p24 protein were calculated by the SoftMax program provided by the manufacturer of the microplate reader. The baseline (day 0) p24 was subtracted from values obtained on later days.
MTT virus infectivity assays.
The cytotoxicity of HIV-1 in the presence and absence of an antiviral agent was correlated to the formation of formazan in an MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay as we have previously described. Day 5 to 7 cultures of MT-2 cells treated without or with antiviral agent and/or other additives and acutely infected with HIV-1 in 96-well microtiter plates were pulsed for 4 h with MTT (1 mg/ml) in RPMI 1640 plus 10% fetal calf serum. Supernatant was removed from cells, and the formazan was solubilized with acid isopropanol (85 μl/well). Absorbance was read at either 540 or 590 nm using a Molecular Dynamics plate reader. Means ± standard deviations (SDs) of triplicate wells were used for calculation of cytotoxicity or inhibition of HIV-1-mediated apoptosis. Means ± SDs of four replicates were used for calculation of cell viability within the combination matrix.
Sensitivity of viral isolates to abacavir.
The sensitivity of the virus isolated from the hollow fiber at the end of an exposure to abacavir was determined by dose-response determinations in an MTT-based cytotoxicity assay. In order to remove residual drug from the virus preparation, the samples from the ECS of the hollow fiber were dialyzed for 4 h against two changes of a 200-fold volume of cold serum-free medium. The virus was aliquoted and stored until the results of titration of the virus were performed. Briefly, MT-2 cells were infected with serial 1:2 dilutions of the dialyzed cell supernatant removed from the ECS at the end of the hollow-fiber study. When the results of this titration were available, we set up a dose-response experiment. MT-2 cells were infected at 106 cells per ml with a dilution of each isolate to be tested that was able to produce adequate cytopathic effects within the 5- to 7-day assay period (usually 2,000 50% tissue culture infective doses). After 1 h cells were centrifuged free of the virus and the cells were suspended at 105. One hundred microliters of cells was added to the central 60 wells of a microtiter plate that had previously received 100 μl of a twofold concentration of the drug in growth medium. Twofold serial dilutions of the drug were set horizontally, resulting in a 2- to 3-log concentration range. Controls included uninfected cells, untreated wells, and cells infected with a virus(es) of known susceptibility to the drug in question.
Genotypic analysis.
For experiments in which genotypic (and phenotypic) analysis was performed, the molecularly cloned HXB-2 strain of HIV-1 was used. Sequencing of the reverse transcriptase (RT) region was performed by the SRA Life Sciences sequencing group after RNA extraction and PCR. Viral RNA was extracted using the QIAamp Viral RNA kit (Qiagen, Valencia, Calif.). Half of the RNA extracted was used to generate HIV cDNA encompassing both the RT and protease gene regions. One to two microliters of the first PCR product was specifically amplified for the RT and/or protease bands using a nested PCR procedure with Amplitaq Gold Polymerase (Perkin-Elmer, Palo Alto, Calif.) to ensure fidelity. PCR products were visualized and quantified on agarose gels against known standards. ABI Prism Big Dye chemistry was used in the sequencing reactions, which were loaded and run on an ABI 377XL automated sequencer (PE Applied Biosystems, Foster City, Calif.). The resulting data were individually analyzed for proper tracking and base-calling, and the completed sequence was used to generate a mutational analysis report.
RESULTS
Anti-HIV activity and protein binding.
We have shown that α1 acid glycoprotein (α1-AGP) can bind to and markedly (>10-fold) reduce the anti-HIV activity of highly protein bound HIV-1 protease inhibitors (3). We evaluated the effect of physiological concentrations of α1-AGP and albumin alone and in combination on the anti-HIV-1 activity of abacavir in vitro. When the MT-2 cell line was infected with HIV-1 strain IIIB in the presence of increasing concentrations of abacavir, a sharp dose-response curve was established. There was a modest difference in the curve when the growth media were supplemented with human serum proteins. It should be pointed out that human serum proteins, particularly albumin, have an enhancing effect upon cell growth and the extent of inhibition of growth caused by an antiretroviral agent can only be evaluated on a percent control basis. Importantly, similar findings were demonstrated with CEM cells infected with HIV-1 strain MN. In these experiments viral inhibition was measured by p24 assay. There is at most a 1.5-fold change in the 50% effective concentrations (EC50s) and EC95s in the presence of physiological concentrations of albumin or α1-AGP. These data suggested to us that serum protein binding would have a little to no effect on the anti-HIV-1 activity of abacavir, indicating that it would not be necessary to perform hollow-fiber experiments in the presence of serum proteins.
Computer simulation of human pharmacokinetic data.
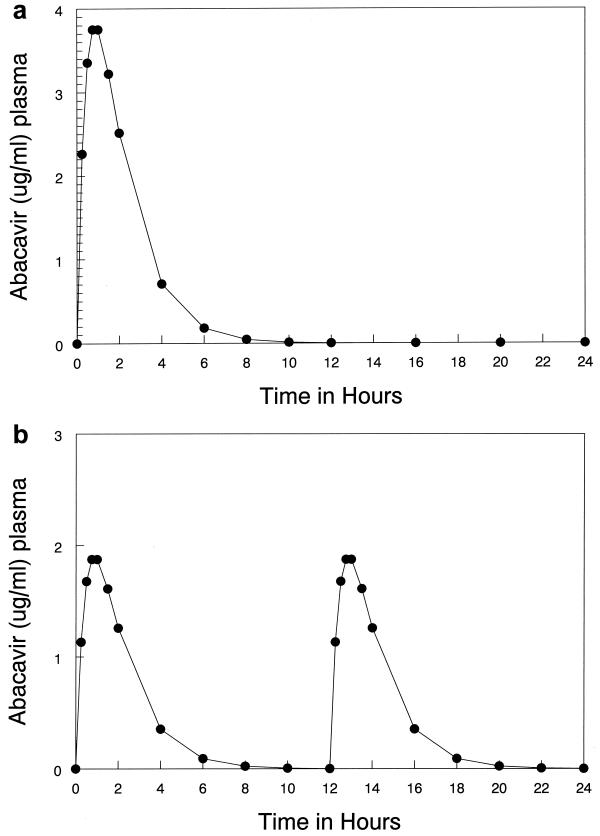
Preliminary experiments indicated that a continuous-infusion suppression exposure was approximately four times the viral EC50. Calculation of an AUC for a steady-state 24-h period (AUC24SS) allowed us to choose 500 mg per day as the best oral exposure which would approximate this AUC. Multidose pharmacokinetic data from phase I/II trials of abacavir were modeled using the programs in the ADAPT II software package (user's guide; Biomedical Simulations Resource, Los Angeles, Calif.). The actual dose modeled that provided the estimates for the parameter values was 300 mg, orally, administered q12h. A typical simulation of the human pharmacokinetics for a 500-mg oral dose is shown in Fig. 1A.As can be seen the peak level in plasma was reached after approximately 45 min and was maintained for approximately 15 min, followed by a decline in drug concentration, with a calculated half-life of approximately 1 h. The AUC24SS for the 500-mg dose was determined to be 10.08 mg·h/liter. An equivalent exposure is shown in Fig. 1B (250 mg q12h orally). The mean concentration in plasma calculated for a continuous infusion of abacavir was 0.42 μg/ml (0.42 μg/ml for 24 h). This concentration would be in excess of the EC95 and would be expected to control the spread of the virus as we determined in our initial dose response experiments.
FIG. 1.
Computer simulation of a 500-mg oral dose. Multidose pharmacokinetic data were modeled using the programs in the ADAPT II software package. The points are the computer-simulated weighted means for the patient population. The AUC24 for the 500-mg dose was determined to be 10.08 mg · h/liter. (A) Simulated profile for 500 mg administered once daily. (B) Simulated profile for 250 mg administered q12h.
Hollow-fiber pharmacodynamic studies.
The initial series of experiments were devised to compare the 500-mg dose given as a continuous infusion to the same dose given once a day. One bioreactor was cultured in the absence of the drug in a continuous-infusion loop where 200 ml of medium was circulated through the hollow fibers at a constant rate. A second hollow-fiber bioreactor was exposed to abacavir at a concentration of 0.42 μg/ml with a similar 200-ml continuous-infusion loop. Medium was exchanged on a daily basis to remove metabolites and prevent exhaustion or decay of the drug (despite the observation that the drug was stable and the medium was capable of providing for adequate cell growth for several days). In parallel to the constant infusion loops, an in vitro pharmacokinetic model system was used to expose cells in the hollow-fiber bioreactor to fluctuating drug concentrations. In the model, drug was introduced into the dosing port of the 100-ml central compartment so that the drug concentration increased to a level of 3.75 μg/ml over a period of 45 min. The drug was allowed to hold at that concentration for a period of 15 min, at which time the drug level in the central compartment was reduced by one-half each hour to simulate a 1-h half-life.
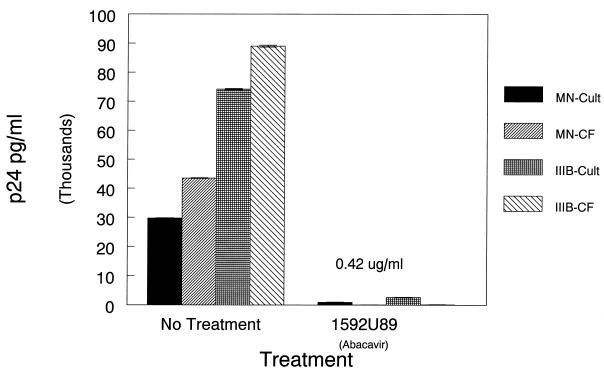
While traditional studies comparing the dose response to drugs involve de novo infections with cell-free virus, our hollow-fiber studies involve spread of the virus from chronically infected to uninfected cells, which involves cell-cell fusion as well as virus-cell fusion. EC50s and EC90s determined in the traditional way are usually similar but are not identical to concentrations that limit HIV-1 spread in the hollow-fiber culture. As we described above, computer simulation of human pharmacokinetics of a 500-mg oral dose would provide a mean concentration in plasma of 0.42 μg/ml as a continuous infusion. We then compared de novo infection with HIV-1 strains IIIB and MN with a coculture of CEM and H9 infected with IIIB or MN (100 uninfected to 1 infected cell). Figure 2shows the results of an experiment in tissue culture flasks using strains HIV-1 MN and IIIB wherein cells were treated with abacavir at a constant exposure of 0.42 μg/ml for a period of 1 week. Examination of the treated cocultures showed a marked reduction in unintegrated HIV DNA as determined by PCR (data not shown).
FIG. 2.
Comparison of the efficacy of abacavir on cell infection of CEM cells with HIV strains IIIB and MN versus cocultivation of CEM cells with HIV-infected H9 cells at a 100:1 ratio. The dose of drug was 0.42 μg/ml, which was the calculated value for a constant exposure of cells to the simulated 500-mg dose. HIV p24 was measured in cell supernatants of the cultures at day 7 postinfection.
Figure 3ashows a hollow-fiber experiment in which a mixture of HIV-1 strain IIIB-infected cells were cocultivated with uninfected CEM cells at a ratio of 1:100. This hollow-fiber pharmacodynamic experiment was designed to compare the 500-mg dose given as a continuous infusion to the same dose given once a day. Computer-driven pumps that controlled both the drug input into and the dilution of the central (plasma) compartment were used to simulate the human pharmacokinetic profile for the once-a-day (q24h) dose. Analysis of HIV-1 spread by measurement of HIV p24 in the cell medium indicated that the simulated q24h dose was as effective as constant exposure to 0.42 mg/ml in limiting HIV-1 replication over the 15 days that the culture was monitored. As can be seen in Fig. 3a, p24 in the untreated culture rapidly reached a point when there was an exponential increase in HIV p24. In contrast the hollow-fiber cultures exposed to abacavir had a slow rise in the level of p24. Since abacavir does not inhibit the release of the virus from previously infected cells, the rise in p24 most likely is the result of an accumulation of viruses released from the chronically infected H9 cells within the culture. To prove whether this was the case we analyzed cultures for the presence of unintegrated HIV DNA. Previous studies from our laboratory had indicated this was a useful marker for de novo replication since unintegrated HIV DNA occurs early in the replication cycle. Analysis of circles with one and two long terminal repeats indicated that viral replication was occurring only at very low concentrations of abacavir. These data suggest that chronically infected cells persist in treated cultures, but spread of the infection is markedly reduced both by continuous or q24h exposure to a simulated 500-mg dose of abacavir.
FIG. 3.
(a) Effect of abacavir on the spread of HIV strain IIIB. Three bioreactors were set with 3 × 107 CEM cells and 3 × 105 H9 IIIB cells (1:100 I/u). One hollow-fiber bioreactor was exposed to the calculated constant infusion level of a 500-mg dose (0.42 μg/ml abacavir) (▴). The second was exposed to a simulated 500-mg q24h dose (•) using the computer-driven pump to simulate an oral dose. The third was an untreated unit (▪) that was continuously exposed to medium without drug. Samples were taken at the indicated time points, and p24 was measured as indicated. (b) Effect of abacavir on the spread of HIV strain MN. Three bioreactors were set with 3 × 107 CEM cells and 3 × 105 H9 MN cells (1:100 I/u). One hollow-fiber bioreactor was exposed to the calculated constant infusion level of a 500-mg dose (0.42 μg/ml abacavir) (▴). The second was exposed to a simulated 500-mg q24h dose (•) using the computer-driven pump to simulate an oral dose. The third was an untreated unit (▪) that was continuously exposed to medium without drug. Samples were taken at the indicated time points and p24 was measured as indicated. (c) Effect of the schedule on the ability of the same total exposure of abacavir (AUC24 = 10.0838 mg·h/liter) on the spread of HIV strain MN. Three bioreactors were set with 3 × 107 CEM cells and 3 × 105 H9 MN cells (1:100 I/u). One hollow-fiber bioreactor was exposed to the 250-mg q12 dose (▪). The second was exposed to a simulated 500-mg q24h dose (▴) using the computer-driven pumps to simulate an oral dose. The third was an untreated unit (•) that was continuously exposed to medium without drug. Samples were taken at the indicated time points, and p24 was measured as indicated.
Given the potential clinical importance of this finding we attempted to repeat this experiment using HIV-1 strain MN. We had previously had good success with HIV-1 strain MN-infected H9 cells in hollow-fiber cultures, albeit virus production and spread were not as rapid as those we have obtained with HIV-1 strain IIIB-infected H9 cells. As can be seen in Fig. 3b, both continuous dosing with 0.42 μg/ml and the computer-simulated 500-mg q24h oral doses resulted in marked suppression of HIV-1 strain MN replication. Nonetheless, it is easy to see that the extent of viral spread in these cultures was substantially lower than we have observed in earlier cultures where HIV-1 strain IIIB was used.
We then proceeded to perform a dose fractionation experiment in which we compared cocultures (CEM and H9 HIV-1 MN) exposed to abacavir given as simulated 250-mg twice-daily (q12h) or 500-mg daily doses. The results are shown in Fig. 3c. Both schedules effectively limited HIV spread. A phenotypic analysis of virus sensitivity to abacavir after exposure for 14 days in the hollow-fiber system at 500 mg q24h or 250 mg q12h showed that the sensitivity of the virus produced in the system was wild type (EC50s of 0.238 and 0.241, respectively).
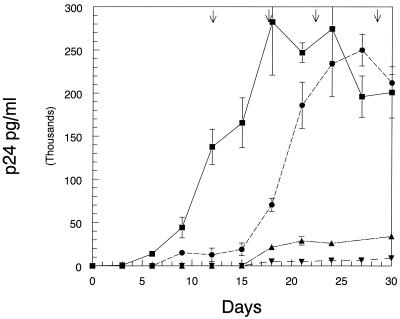
The potential for a large number of replication cycles exists in the hollow-fiber system due to the near-tissue density of susceptible uninfected cells and the relatively high proportion (1%) of virus-producing chronically infected cells in the long-term cultures. Theoretically, under selection pressure, marginally effective abacavir regimens may fail because resistant viruses within the population might emerge with longer times in coculture. Therefore, we examined the clinical dose of 600-mg per day in three regimens producing the same AUC24: 300 mg q12h, 600 mg q24h, and 1,200 mg q48h. HIV-1 strain HXB-2, which was derived from a molecular clone, was used for these studies. In preliminary studies, this virus was sequenced at baseline and after 30 days of expansion in hollow-fiber culture, and only four alterations from the HIV-1 consensus sequence of the RT region were observed at both time points. This is indicative that the RT gene sequence was stable for at least the 30 days required to perform analysis of viral populations exposed to the 600-mg/day dose of abacavir. The result of this experiment, which was run for 30 days, is displayed in Fig. 4.While the 1,200-mg q48h regimen delayed viral replication initially, it is not different from control by 30 days. The q24h and q12h regimens demonstrate consistent viral suppression. It is also evident that the once-daily regimen had a small increase in viral output at day 18, but this unit then remained stable out to day 30. Virus from each of the hollow-fiber cultures was subjected to both phenotypic and genotypic analysis. Again, the abacavir sensitivity of the virus at day 30 was phenotypically and genotypically wild type. Genotype analysis of the RT region revealed four alterations from the consensus HIV-1 sequence in all treated and untreated groups. No mutations related to abacavir resistance were observed in the virus populations analyzed.
FIG. 4.
Examination of the effect of no therapy and abacavir (300 mg q12h, 600 mg q24h, and 1,200 mg q48h) on the spread of HIV strain HXB-2. Samples for p24 analysis were from cell supernatant fluid from the ECS of individual hollow-fiber units and frozen at −70°C until the samples were taken at the 15- and 30-day points. At day 15 and day 30 samples from each hollow fiber were thawed, diluted, and tested in a p24 antigen capture enzyme-linked immunosorbent assay. The means of two determinations and the SDs are plotted on a linear scale.
DISCUSSION
Only in the last several years has it become clear that HIV disease may become a chronic disease in which patients may live for many years and during which a high quality of life can be maintained. This major change is due to the advent of very active drugs for the therapy of the disease. However, it has also become clear that protease inhibitor monotherapy is inadequate, as large numbers of patients will experience emergence of viral resistance, even in a relatively short time span, such as 24 weeks (4, 6).
It has also become clear that two elements are involved in suppression of the emergence of HIV-1 resistance. The first is the ability of a regimen to markedly inhibit HIV-1 replication (i.e., reduce the plasma HIV-1 RNA copy number to below the level of detection of the current assays), and the second is combination chemotherapy which requires the virus to mutate at multiple sites (4). Adherence also plays a major role, as continuously taking medicines on a frequent (q6h basis or even more frequently) basis cannot be maintained for long periods by a majority of a population. Failure to maintain inhibitory levels of drug in the face of persistent cellular reservoirs of infection will possibly lead to increased emergence of resistance.
For all these reasons, the advent of additional agents with potent activity which could be administered infrequently (q12h or q24h) would be welcome, indeed.
Our intent here was to examine, using our hollow-fiber pharmacodynamic model system, the most likely adequate daily dose of such a drug (abacavir) and to ascertain the impact of schedule of administration on the activity of the drug. We also wanted to examine other factors, such as protein binding, which we have previously shown to adversely affect antiretroviral activity, at least in the case of protease and nonnucleoside RT inhibitors.
Unlike protease inhibitors, purified plasma proteins in physiological amounts have no major effect on the activity of abacavir.
Work at Glaxo-Wellcome (Margaret Tisdale, personal communication) has demonstrated that the EC95 of the IIIB strain is higher than the median for populations of clinically derived strains of HIV-1. Consequently, we wished to also examine the MN strain, which better approximates the median value of EC95.
Figure 3a demonstrates that an abacavir concentration of 0.42 μg/ml, which is four times the EC50 and which approximates the EC95 for strain IIIB, is quite effective in limiting the spread of the infection from the chronically infected cells of the challenge to uninfected cells when either p24 in the cell medium or unintegrated viral DNA expression is used as a measure of effect. This concentration of drug was used in a scenario in which continuous drug infusion was simulated. This produced a 24-h exposure of 10.08 μg · h/ml (0.42 μg/ml for 24 h). When this exact same drug exposure (equivalent AUCSS to 500 mg of abacavir administered as 250 mg q12h in clinical trials) was simulated as a once-daily oral administration profile with a 1-h terminal half-life, it provided equivalent suppression of viral spread as the continuous-infusion profile (Fig. 3a). Such a finding would indicate that it is the AUC (relative to some measure of viral potency, such as EC50 or EC95) that drives antiviral effect for abacavir.
Examination of Fig. 3b and c, employing the MN strain of HIV-1, supports the same conclusion. Comparison of once-daily and continuous-infusion regimens (Fig. 3b) and once-daily versus twice-daily administration regimens also demonstrates equivalent inhibition of viral spread.
Because of the clinical implications of proceeding to clinical trial with a schedule that might promote the emergence of resistance, we decided to extend the experiments to 30 days of monotherapy, to use the clinically approved dose of abacavir (600 mg/day), and to examine a regimen that would lead to inadequate viral suppression at the end of the dosing interval. To this end, we examined dose schedules of 300 mg q12h, 600 mg q24h, and 1,200 mg q48h. A molecularly cloned virus (HIV-1 strain HXB-2) was chosen for these studies and genotyped prior to the experimental series. Clearly, viral suppression subsequent to the once-daily exposure was quite close to that of the q12h regimen, except for a small increase in viral output at day 18, which did not increase thereafter. The q48h schedule showed virtually no viral suppression, compared to untreated control. Since there is an upper limit on the capacity of the bioreactor (circa 1010 cells/ml) we removed cells from the bioreactors at the indicated time points (Fig. 4). This process is not quantitative, albeit we performed the same protocol at the same time for each treatment reactor, and may explain the increased expression of virus at the later time points. Sampling the hollow-fiber units at day 30 and determining phenotypic sensitivity to abacavir demonstrated that isolates from all regimens gave wild-type results for the EC50. This was concordant with the genotypic analysis which indicated no mutations relevant to abacavir sensitivity of the isolates.
Faletto and colleagues at Glaxo-Wellcome have reported that the half-life for carbovir triphosphate is on the order of 3.4 h (5). This makes the seeming equivalence of 250 to 300 mg q12h with 500 to 600 mg q24h suspect. However, it should be recognized that the necessity for production of triphosphorylated carbovir (the active form of abacavir) may also be an advantage. It is important that experiments with another nucleoside that also has a 3- to 4-h intracellular half-life of the triphosphate anabolite (zidovudine) did not support the efficacy of a once-a-day dosing regimen (data not shown). How could one explain the apparent efficacy of once-a-day abacavir? In one scenario, if a Michaelis-Menten step is present, then the rate of fall of the pre-Michaelis-Menten form toward the Km of the enzyme would determine the period during which the triphosphate form would have stable concentrations, even in the absence of parent compound abacavir external to the cell. Unfortunately, there is currently little evidence of a Michaelis-Menten step in the anabolism of abacavir to carbovir triphosphate.
It is important to understand how triphosphate half-life estimates are generated in order to produce another hypothesis to explain the present findings. Cells are loaded for a prolonged period of time with the nonnucleoside RT inhibitor in question until intracellular steady state of all forms is achieved. The external drug is then completely removed (which is nonphysiological), and the decline in the triphosphate moiety is then monitored.
In the clinic, there is a concentration of parent drug present that continuously declines but is continuously present. Whatever drug is present produces some rate constant for phosphorylation that opposes the decline in mono-, di-, and triphosphate forms of the drug. This means that the half-life of decline estimated from the traditional in vitro experiment grossly underestimates the true decline in vivo. Further, there may be a postantiviral effect engendered that would be the analogue of the postantibiotic effect seen with bacteria. However, it is also possible to run out of persistent effects when schedules of administration produce intervals of administration that are too long. This was seen in animal experiments for aminoglycosides by Vogelman and colleagues (7). Here, however, it is clear that once-daily abacavir effectively suppresses viral replication in vitro.
These in vitro data suggest that 500 mg of abacavir a day (AUC24 = 10.08 mg · h/liter) can effectively limit HIV-1 infection when given as a 250-mg q12h or 500-mg q24h dose. One should realize that the clinical dose of abacavir chosen for clinical trial evaluation is 300 mg administered orally q12h. Obviously, this produces a daily AUC 20% greater than that simulated here. It is also clear that this in vitro system is not meant to be a substitute for clinical trial data that would directly compare doses and schedules. Obviously the exposures modeled here represent optimal exposures with perfect adherence, a best-case scenario. The system does not take into account virus that can be replicating at privileged sites or failure of the drug to penetrate certain compartments. It will, however, establish a minimally effective drug concentration and suggest whether a schedule could be successful. We feel that these data show that once-daily administration of abacavir may be possible, particularly as part of a combination regimen.
A clinical trial evaluation of abacavir employed on a once-daily basis (trial EPV 40001) has been published (C. Bowonwatanuwong, P. Mootsikapun, K. Supparatpinyo, S. Tansuphaswadikul, R. Athisegran, P. Pasook, and A. Jones, 1st Int. AIDS Soc. Conf. HIV Pathog. Treatment, abstr. 4, 2001) and indicates that once-daily therapy with this agent is feasible.
Acknowledgments
We thank Roger Sparks (SRA Life Sciences), who performed many of the HIV p24 assays, and Kimberly George, who analyzed the genotypic data. The support of scientific and technical staff at SRA Life Sciences is gratefully acknowledged.
REFERENCES
- 1.Bilello, J. A., G. Bauer, M. N. Dudley, G. A. Cole, and G. L. Drusano. 1994. Effect of 2",3"-didehydro-3"-deoxythymidine in an in vitro hollow-fiber pharmacodynamic model system correlates with results of dose ranging clinical studies. Antimicrob. Agents Chemother. 38:1386-1391. [DOI] [PMC free article] [PubMed]
- 2.Bilello, J. A., P. A. Bilello, J. J. Kort, M. N. Dudley, J. Leonard, and G. L. Drusano. 1995. Efficacy of constant infusion of A-77003, an inhibitor of the human immunodeficiency virus type 1 (HIV-1) protease, in limiting acute HIV-1 infection in vitro. Antimicrob. Agents Chemother. 39:2523-2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bilello, J. A., P. A. Bilello, K. Stellrecht, J. Leonard, D. Norrbeck, D. J. Kempf, T. Robbins, and G. L. Drusano. 1996. Human serum alpha 1 acid glycoprotein reduces uptake, intracellular concentration, and antiviral activity of A-80987, an inhibitor of the human immunodeficiency virus type 1 protease. Antimicrob. Agents Chemother. 40:1491-1497. [DOI] [PMC free article] [PubMed]
- 4.Drusano, G. L., J. A. Bilello, D. S. Stein, M. Nessly, A. Meibohm, E. A. Emini, P. Deutsch, J. Condra, J. Chodakewitz, and D. J. Holder. 1998. Factors influencing the emergence of resistance to indinavir: role of virologic, immunologic, and pharmacologic variables. J. Infect. Dis. 178:360-367. [DOI] [PubMed] [Google Scholar]
- 5.Faletto, M. B., W. H. Miller, E. P. Garvey, M. H. St. Clair, S. M. Daluge, and S. S. Good. 1997. Unique intracellular activation of the potent anti-human immunodeficiency virus agent 1592U89. Antimicrob. Agents Chemother. 41:1099-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kempf, D. J., R. A. Rode, Y. Xu, E. Sun, M. E. Heath-Chiozzi, J. Valdes, A. J. Japour, S. Danner, C. Boucher, A. Moller, and J. M. Leonard. 1998. The duration of viral suppression during protease inhibitor therapy for HIV-1 infection is predicted by plasma HIV-1 RNA at the nadir. AIDS 12:F9-F14. [DOI] [PubMed]
- 7.Vogelman, B., S. Gudmundsson, J. Leggett, J. Turnidge, S. Ebert, and W. A. Craig. 1988. Correlation of antimicrobial pharmacokinetic parameters with therapeutic efficacy in an animal model. J. Infect. Dis. 158:831-847. [DOI] [PubMed] [Google Scholar]