Abstract
Mycobacterium tuberculosis and M. avium complex strains given intramacrophage passage (I-type) were compared with those cultured in a liquid medium (E-type) for their drug susceptibilities when they were replicating in Mono-Mac-6 macrophages or A-549 cells. Their intracellular susceptibilities to rifalazil, clarithromycin, and levofloxacin were decreased more in I-type organisms than in E-type organisms, except that their rifalazil susceptibility inside A-549 cells was markedly increased in I-type organisms.
Mycobacterium avium complex (MAC) adapted to intracellular milieus by intramacrophage passage (I-type) is known to display increased efficiency in invading other macrophages compared to extracellularly grown MAC (E-type) (2). It is also reported that Mycobacterium tuberculosis obtained by intramacrophage passage (I-type) more efficiently invades type II pneumocytes, one of the major portals of mycobacterial entry to the lungs (1, 7, 9, 10), and exerts greater cytotoxicity than extracellularly passaged M. tuberculosis (E-type) (6). It thus appears that the mode of interaction of I-type mycobacteria with macrophages and type II pneumocytes is different from that of E-type organisms. Therefore, we examined the intracellular susceptibilities of I-type and E-type mycobacteria to some antimicrobials, including rifalazil (RLZ) (3, 11), clarithromycin (CLR), and levofloxacin (LVX), when these organisms were residing within macrophages or type II pneumocytes.
M. tuberculosis Kurono and MAC N-444 (M. avium, serovar 8) strains, grown in 7H9 broth or passaged through Mono-Mac-6 (MM6) human monocytic cells (12) for 5 days, were used as E-type or I-type organisms, respectively. The intracellular susceptibilities to the test drugs of these organisms inside MM6 macrophages or A-549 human type II alveolar cells were measured as follows (10). First, MM6 macrophages cultured in RPMI 1640 medium (RPMI) containing 5% fetal bovine serum (FBS) were infected with M. tuberculosis (multiplicity of infection [MOI] = 3) or MAC (MOI = 10) at 37°C in a CO2 incubator for 4 h. After being washed with 2% FBS-Hanks' balanced salt solution by centrifugation (150 × g, 5 min), infected cells (4 × 104) were cultivated in 1% FBS-RPMI (0.2 ml) in the presence or absence of test drugs at the maximum concentration of drug in serum (Cmax) (Fig. 1).At intervals, the cells were lysed with 0.07% sodium dodecyl sulfate, subsequently neutralized with 6% bovine serum albumin, and released organisms were collected and washed with distilled water by centrifugation (2,000 × g, 30 min). The CFU of recovered organisms were counted on 7H11 agar plates. Second, A-549 cells (4 × 104) cultivated in 5% FBS-Ham's F-12K medium were infected with M. tuberculosis (MOI = 3) or MAC (MOI = 10) at 37°C for 3 h. After being washed with 2% FBS-HBSS, infected cells were cultured in 0.2 ml of 1% FBS-F-12K medium in the presence or absence of test drugs at Cmax. At intervals, a CFU counting assay was done as described above.
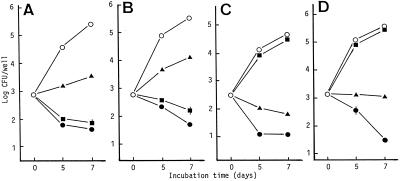
FIG. 1.
Antimicrobial activities of RLZ (•), CLR (▴), and LVX (▪) against E-type (A) and I-type (B) M. tuberculosis and E-type (C) and I-type (D) MAC replicating within MM6 macrophages. Test drugs were added at the Cmax in blood after oral administration at clinical or subclinical dosages: RLZ (2 mg/kg) (3), 0.05 μg/ml; CLR (10 mg/kg), 2.3 μg/ml; LVX (5 mg/kg), 2.0 μg/ml. Each plot indicates the mean ± standard error (n = 3; error bars omitted for values <0.1). The results are representative of two independent experiments. By the broth dilution method with 7HSF medium, MICs of RLZ, CLR, and LVX for E-type M. tuberculosis were 0.003, 25, and 0.4 μg/ml, and those for I-type M. tuberculosis were 0.0015, 25, and 0.4 μg/ml, respectively. MICs of these drugs for E-type MAC were 0.013, 6.25, and 12.5μg/ml, and those for I-type MAC were 0.025, 6.25, and 12.5 μg/ml, respectively.
I/E[inv], the ratio of invasiveness of I-type organisms to that of E-type organisms, was calculated as (intracellular CFU of I-type organisms after infection)/(intracellular CFU of E-type organisms after infection). I/E[growth], the ratio of intracellular growth of I-type organisms to that of E-type organisms, was calculated as (ΔCFU of I-type organisms during a 5-day cultivation)/(ΔCFU of E-type organisms during a 5-day cultivation). I/E[Drug-S], the ratio of the decrease in residual CFU of I-type organisms due to the activity of test antimicrobial agents to that of E-type organisms, was calculated as [CFUI-type (−drug)/CFUI-type(+drug)]/[CFUE-type(−drug)/CFUE-type(+drug)].
Figure 1 shows the antimicrobial effects of test drugs against E-type and I-type mycobacteria residing within MM6 macrophages. First, I/E[inv] values of M. tuberculosis and MAC were 0.79 and 4.17, respectively. Thus, invasiveness of M. tuberculosis into MM6 macrophages was decreased by intramacrophage passage, whereas the opposite result was obtained for MAC. I/E[growth] values of M. tuberculosis and MAC were 2.81 and 2.29, respectively, indicating that their ability to replicate within MM6 macrophages was increased by intramacrophage passage. Second, RLZ and LVX exhibited CFU-reducing activity, and CLR caused growth inhibition of both types of M. tuberculosis. Notably, the intracellular susceptibility of I-type M. tuberculosis to these drugs was somewhat lower than that of E-type M. tuberculosis: the mean values of I/E[Drug-S] on days 5 and 7 were 0.85, 0.48, and 0.57 for RLZ, CLR, and LVX, respectively. Third, RLZ reduced bacterial CFU of both types of MAC. CLR also reduced bacterial CFU of E-type MAC, but it caused only growth inhibition of I-type MAC. LVX showed weak antimicrobial action against them. In most cases, the intramacrophage drug susceptibility of I-type MAC was somewhat lower than that of the E-type MAC: I/E[Drug-S] values were 0.95, 0.71, and 0.71 for RLZ, CLR, and LVX, respectively.
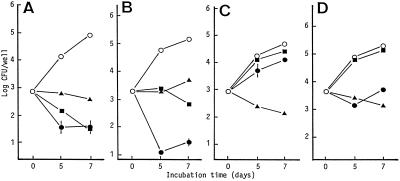
Figure 2shows the results of the same experiment with A-549 cells. First, I-type organisms were more efficient than E-type organisms in invading A-549 cells: I/E[inv] values of M. tuberculosis and MAC were 2.51 and 4.47, respectively. The ability of M. tuberculosis but not MAC to replicate inside A-549 cells was increased by intramacrophage passage: I/E[growth] values of M. tuberculosis and MAC were 1.70 and 0.93, respectively. Second, RLZ exhibited CFU-reducing activity against both types of M. tuberculosis. LVX exerted CFU-reducing activity against E-type M. tuberculosis and showed a bacteriostatic effect against I-type M. tuberculosis. CLR caused growth inhibition of both types of M. tuberculosis. Third, CLR exhibited CFU-reducing activity against both types of MAC, while RLZ caused growth inhibition of them. LVX showed weak inhibitory action against both types of MAC. Fourth, intracellular RLZ susceptibilities of I-type M. tuberculosis and MAC inside A-549 cells were markedly increased compared to those of E-type organisms. In contrast, the susceptibilities of I-type M. tuberculosis and MAC to CLR and LVX were lower than those of E-type organisms: I/E[Drug-S] values of M. tuberculosis were 7.15, 0.77, and 0.31 for RLZ, CLR, and LVX, respectively, and those of MAC were 8.70, 0.61, and 0.90 for these drugs, respectively.
FIG. 2.
Antimicrobial activities of RLZ (•), CLR (▴), and LVX (▪) against E-type (A) and I-type (B) M. tuberculosis and E-type (C) and I-type (D) MAC replicating within A-549 cells. Test drugs were added at the Cmax in the blood. The other details are the same as in the legend to Fig. 1.
Concerning the present findings, the following discussion can be made. First, this study indicated that the intracellular susceptibilities of M. tuberculosis and MAC to RLZ, CLR, and LVX were significantly altered due to intramacrophage passage of these organisms. This was not the result of changes in the drug susceptibility of test organisms during intramacrophage passage, since MICs of test drugs for E-type and I-type organisms were essentially the same (Fig. 1). Second, it is known that the intracellular fate of mycobacterial organisms is largely dependent on the nature of the intracellular compartments into which they are internalized (8). Indeed, living mycobacteria have the capacity to retain TACO protein at the phagosomal membrane, thereby preventing fusion of the phagosome with lysosomes (5). As reported by Bermudez et al. (2), I-type and E-type mycobacteria may initially bind to macrophage cell membrane via different types of receptors (complement receptors, mannose receptor, vitronectin receptor, β1-integrins, etc.) (4). Intracellular signalling pathways initiated by the ligation of such receptors may differentially influence the maturation of phagosomes and phagolysosomes containing mycobacteria and may regulate the mode of fusion of phagosomal vesicles with the early or late endosomes and lysosomes. Therefore, the difference in the types of macrophage receptors, which are used for mycobacterial entry into macrophages, between I-type and E-type M. tuberculosis or MAC may result in differential efficiencies in the delivery of extracellular antimicrobial drugs into the phagosomes containing these mycobacteria. This hypothesis is being verified by electron microscopy studies of the intracellular delivery of drugs in MM6 macrophages and A-549 cells engulfing the I-type and E-type mycobacteria.
Acknowledgments
We thank Kaneka Corporation, Taisho Pharmaceutical Co., and Daiichi Pharmaceutical Co. for providing RLZ, CLR, and LVX, respectively.
REFERENCES
- 1.Bermudez, L. E., and J. Goodman. 1996. Mycobacterium tuberculosis invades and replicates within type II alveolar cells. Infect. Immun. 64:1400-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bermudez, L. E., A. Parker, and J. R. Goodman. 1997. Growth within macrophages increases the efficiency of Mycobacterium avium in invading other macrophages by a complement receptor-independent pathway. Infect. Immun. 65:1916-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dietze, R., L. Teixeira, L. M. C. Rocha, M. Palaci, J. L. Johnson, C. Wells, L. Rose, K. Eisenach, and J. J. Ellner. 2001. Safety and bactericidal activity of rifalazil in patients with pulmonary tuberculosis. Antimicrob. Agents Chemother. 45:1972-1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ehlers, M. R. W., and M. Daffe. 1998. Interactions between Mycobacterium tuberculosis and host cells: are mycobacterial sugars the key? Trends Microbiol. 6:328-335. [DOI] [PubMed] [Google Scholar]
- 5.Ferrari, G., M. Naito, H. Langen, and J. Pieters. 1999. A coat protein on phagosomes involved in intracellular survival of mycobacteria. Cell 97:435-447. [DOI] [PubMed] [Google Scholar]
- 6.McDonough, K. A., and Y. Kress. 1995. Cytotoxicity for lung epithelial cells is a virulence-associated phenotype of Mycobacterium tuberculosis. Infect. Immun. 63:4802-4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta, P. K., C. H. King, E. H. White, J. J. Murtagh, Jr., and F. D. Quinn. 1996. Comparison of in vitro models for the study of Mycobacterium tuberculosis invasion and intracellular replication. Infect. Immun. 64:2673-2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pieters, J. 2001. Evasion of host cell defense mechanisms by pathogenic bacteria. Curr. Opin. Immunol. 13:37-44. [DOI] [PubMed] [Google Scholar]
- 9.Sato, K., and H. Tomioka. 1999. Antimicrobial activities of benzoxazinorifamycin (KRM-1648) and clarithromycin against Mycobacterium avium-intracellulare complex within murine peritoneal macrophages, human macrophage-like cells and human alveolar epithelial cells. J. Antimicrob. Chemother. 43:351-357. [DOI] [PubMed] [Google Scholar]
- 10.Sato, K., H. Tomioka, T. Akaki, and S. Kawahara. 2000. Antimicrobial activities of levofloxacin, clarithromycin, and KRM-1648 against Mycobacterium tuberculosis and Mycobacterium avium complex replicating within Mono Mac 6 human macrophage and A-549 type II alveolar cell lines. Int. J. Antimicrob. Agents 16:25-29. [DOI] [PubMed] [Google Scholar]
- 11.Tomioka, H. 2000. Prospects for development of new antimicrobial drugs, with special reference to a new benzoxazinorifamycin, KRM-1648. Arch. Immunol. Ther. Exp. 48:183-188. [PubMed] [Google Scholar]
- 12.Ziegler-Heitbrock, H. W. L., E. Thiel, A. Futterer, V. Herzog, and A. Wirtz. 1988. Establishment of a human cell line (Mono Mac 6) with characteristics of mature monocytes. Int. J. Cancer 41:456-461. [DOI] [PubMed] [Google Scholar]