Abstract
1. Flow of carbon atoms from glucose and glycogen glucose to glyceride glycerol, glyceride fatty acids and glycerol was calculated in the perfused rat heart and incubated epididymal adipose tissue from the incorporation of 14C from [U-14C]-glucose (into glyceride glycerol, glyceride fatty acids and glycerol in the medium), and from measurements of the specific activity of l-glycerol 3-phosphate, and the effects of insulin, adrenaline and alloxan-diabetes were studied. Measurements were also made of the uptake of glucose and the outputs of lactate, pyruvate and glycerol. 2. New methods are described for the measurement of radioactivity in small amounts of metabolites (glycerol, glucose 6-phosphate and fructose 6-phosphate and l-glycerol 3-phosphate) in which use has been made of alterations in charge induced by enzymic conversions to effect resolution by ion-exchange chromatography. 3. In hearts the specific activity of l-glycerol 3-phosphate was less than that of glucose in the medium but similar to that of lactate released during perfusion. Because repeated measurements of the specific activity of l-glycerol 3-phosphate was impracticable, the specific activity of lactate has been used as an indirect measurement of glycerol phosphate specific activity. 4. In fat pads, specific activity of lactate was the same as that of glucose in the medium and thus the specific activity of l-glycerol 3-phosphate was taken to be the same as that of medium glucose. 5. In hearts from alloxan-diabetic rats, despite decreased glucose uptake and l-glycerol 3-phosphate concentration, flow of carbon atoms through l-glycerol 3-phosphate to glyceride glycerol was increased about threefold. 6. In fat pads, flow of carbon atoms through l-glycerol 3-phosphate to glyceride glycerol was increased by insulin (twofold), by adrenaline in the presence of insulin (fivefold) and by diabetes in pads incubated with insulin (1·5-fold). These increases could not be correlated either with increases in glucose uptake, which was unchanged by adrenaline and decreased in diabetes, or with the concentration of l-glycerol 3-phosphate, which was decreased by adrenaline and unchanged in diabetes. 7. These results are discussed in relation to the control of glyceride synthesis in heart and adipose tissue and to the regulation of glyceride fatty acid oxidation in the perfused rat heart.
Full text
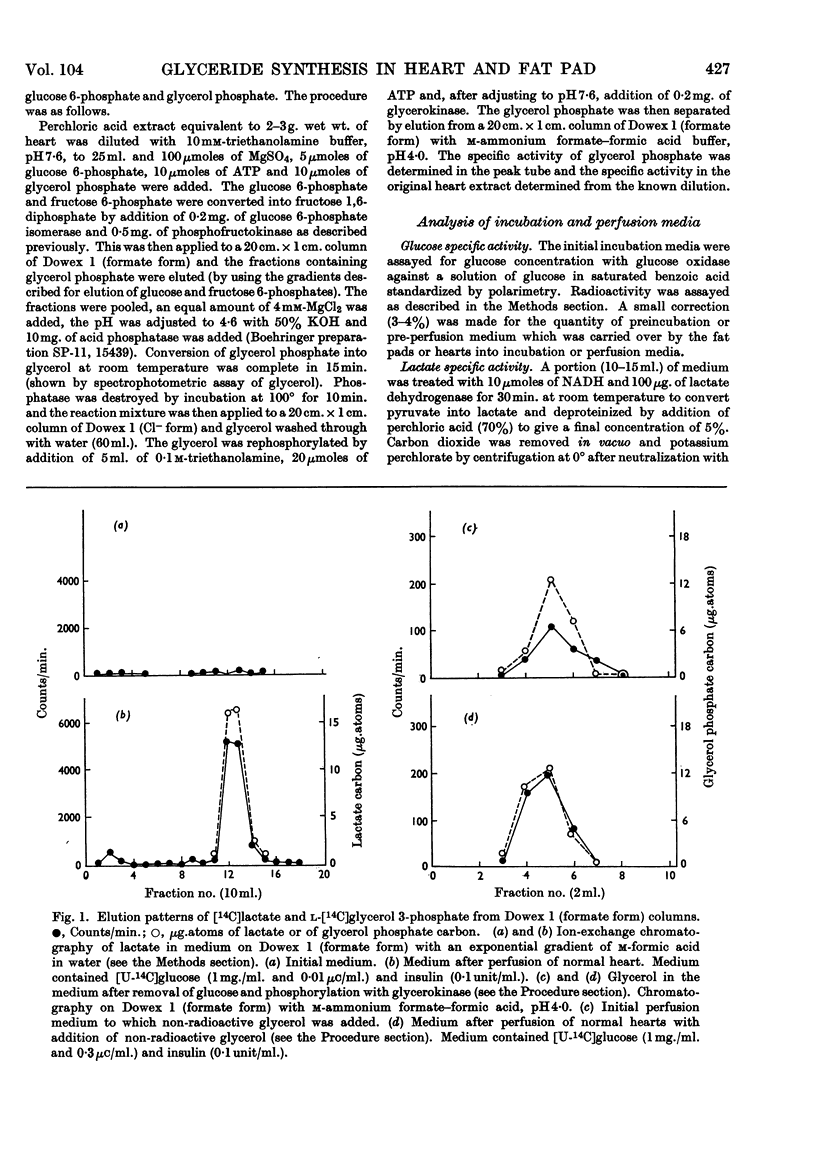
PDF





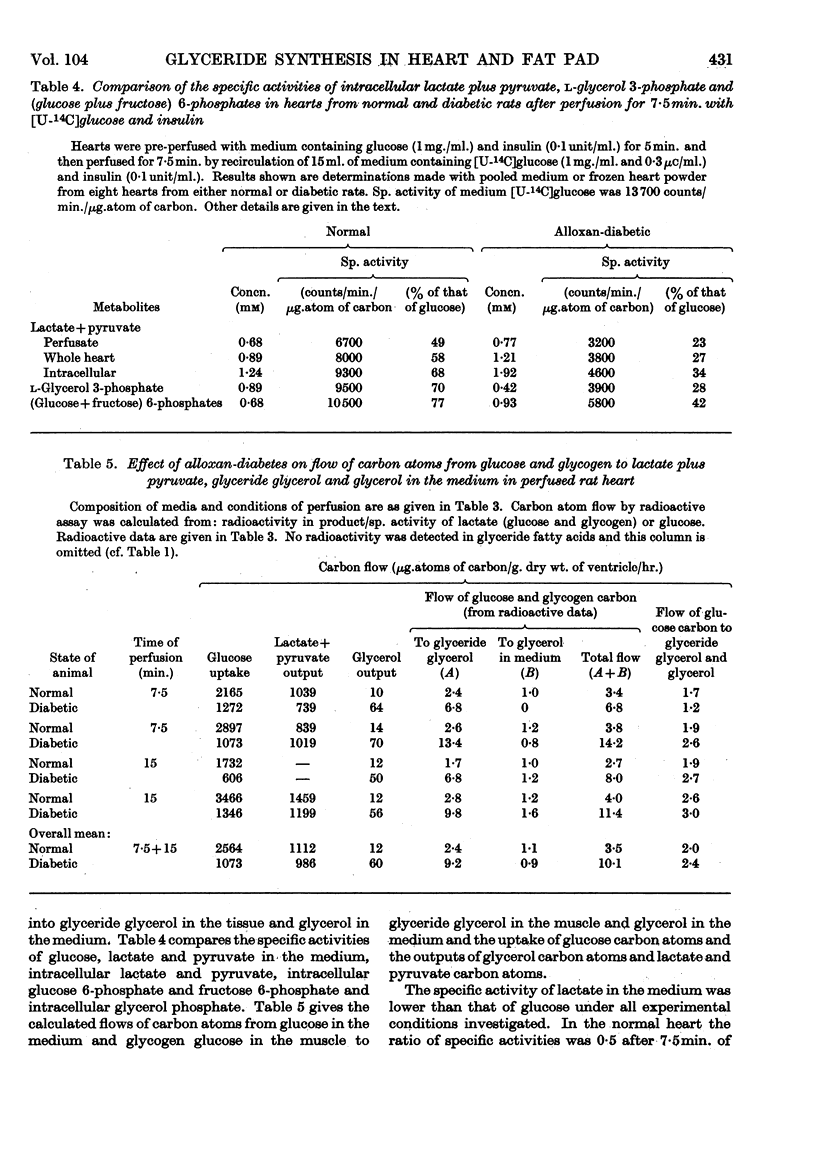



Selected References
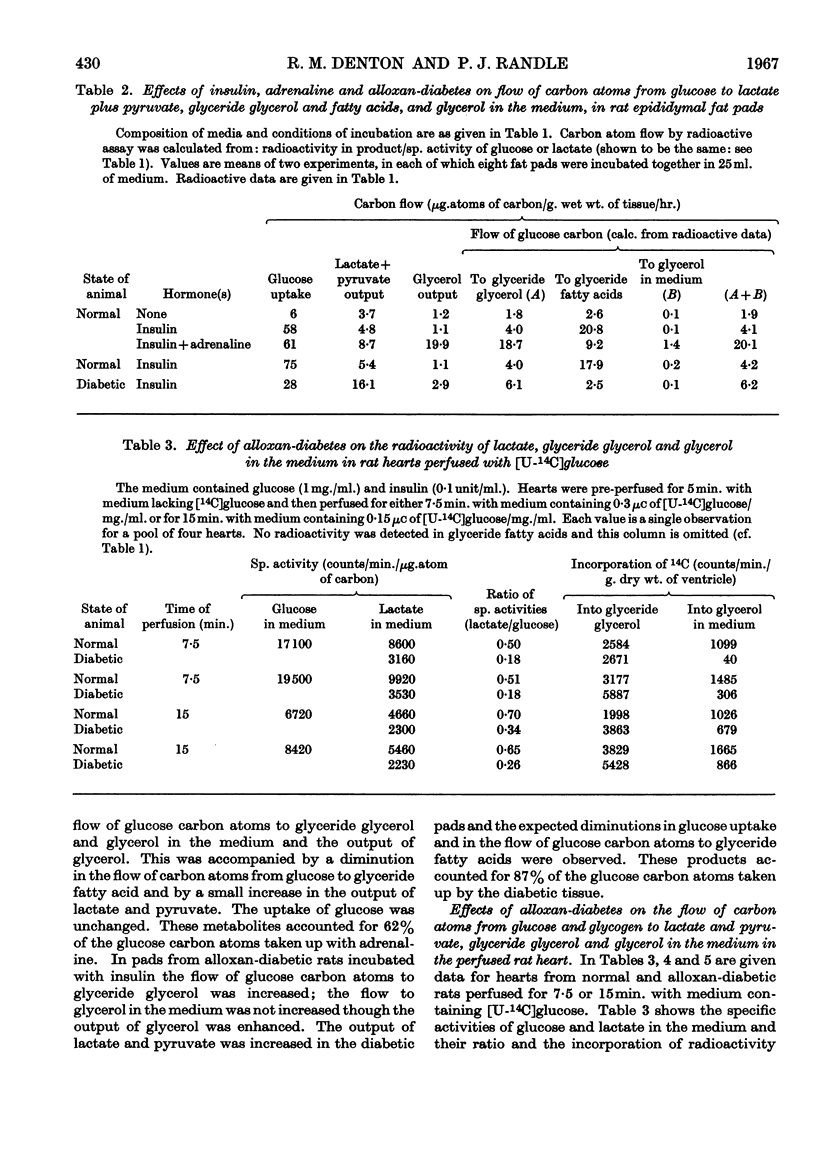
These references are in PubMed. This may not be the complete list of references from this article.
- CAHILL G. F., Jr, LEBOEUF B., FLINN R. B. Studies on rat adipose tissue in vitro. VI. Effect of epinephrine on glucose metabolism. J Biol Chem. 1960 May;235:1246–1250. [PubMed] [Google Scholar]
- CAHILL G. F., Jr, LEBOEUF B., RENOLD A. E. Studies on rat adipose tissue in vitro. III. Synthesis of glycogen and glyceride-glycerol. J Biol Chem. 1959 Oct;234:2540–2543. [PubMed] [Google Scholar]
- CARLSON L. A. DETERMINATION OF SERUM TRIGLYCERIDES. J Atheroscler Res. 1963 Jul-Aug;3:334–336. doi: 10.1016/s0368-1319(63)80012-5. [DOI] [PubMed] [Google Scholar]
- Denton R. M., Yorke R. E., Randle P. J. Measurement of concentrations of metabolites in adipose tissue and effects of insulin, alloxan-diabetes and adrenaline. Biochem J. 1966 Aug;100(2):407–419. doi: 10.1042/bj1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLATT J. P., BALL E. G. STUDIES ON THE METABOLISM OF ADIPOSE TISSUE. XV. AN EVALUATION OF THE MAJOR PATHWAYS OF GLUCOSE CATABOLISM AS INFLUENCED BY INSULIN AND EPINEPHRINE. J Biol Chem. 1964 Mar;239:675–685. [PubMed] [Google Scholar]
- GARLAND P. B., RANDLE P. J. A rapid enzymatic assay for glycerol. Nature. 1962 Dec 8;196:987–988. doi: 10.1038/196987a0. [DOI] [PubMed] [Google Scholar]
- Garland P. B., Randle P. J. Regulation of glucose uptake by muscles. 10. Effects of alloxan-diabetes, starvation, hypophysectomy and adrenalectomy, and of fatty acids, ketone bodies and pyruvate, on the glycerol output and concentrations of free fatty acids, long-chain fatty acyl-coenzyme A, glycerol phosphate and citrate-cycle intermediates in rat heart and diaphragm muscles. Biochem J. 1964 Dec;93(3):678–687. doi: 10.1042/bj0930678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoberman H. D. The fate of the alphah atom of L-lactate in perfused rat liver. Ann N Y Acad Sci. 1965 Jul 31;119(3):1070–1083. doi: 10.1111/j.1749-6632.1965.tb47463.x. [DOI] [PubMed] [Google Scholar]
- Katz J., Landau B. R., Bartsch G. E. The pentose cycle, triose phosphate isomerization, and lipogenesis in rat adipose tissue. J Biol Chem. 1966 Feb 10;241(3):727–740. [PubMed] [Google Scholar]
- Kreisberg R. A. Effect of diabetes and starvation on myocardial triglyceride and free fatty acid utilization. Am J Physiol. 1966 Feb;210(2):379–384. doi: 10.1152/ajplegacy.1966.210.2.379. [DOI] [PubMed] [Google Scholar]
- MORGAN H. E., HENDERSON M. J., REGEN D. M., PARK C. R. Regulation of glucose uptake in muscle. I. The effects of insulin and anoxia on glucose transport and phosphorylation in the isolated, perfused heart of normal rats. J Biol Chem. 1961 Feb;236:253–261. [PubMed] [Google Scholar]
- Newsholme E. A., Randle P. J. Regulation of glucose uptake by muscle. 7. Effects of fatty acids, ketone bodies and pyruvate, and of alloxan-diabetes, starvation, hypophysectomy and adrenalectomy, on the concentrations of hexose phosphates, nucleotides and inorganic phosphate in perfused rat heart. Biochem J. 1964 Dec;93(3):641–651. doi: 10.1042/bj0930641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OPIE L. H., EVANS J. R., SHIPP J. C. EFFECT OF FASTING ON GLUCOSE AND PALMITATE METABOLISM OF PERFUSED RAT HEART. Am J Physiol. 1963 Dec;205:1203–1208. doi: 10.1152/ajplegacy.1963.205.6.1203. [DOI] [PubMed] [Google Scholar]
- Randle P. J., Newsholme E. A., Garland P. B. Regulation of glucose uptake by muscle. 8. Effects of fatty acids, ketone bodies and pyruvate, and of alloxan-diabetes and starvation, on the uptake and metabolic fate of glucose in rat heart and diaphragm muscles. Biochem J. 1964 Dec;93(3):652–665. doi: 10.1042/bj0930652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHAPIRO B., CHOWERS I., ROSE G. Fatty acid uptake esterification in adipose tissue. Biochim Biophys Acta. 1957 Jan;23(1):115–120. doi: 10.1016/0006-3002(57)90292-5. [DOI] [PubMed] [Google Scholar]
- SHORT D. J., PARKES A. S. Feeding and breeding of laboratory animals; a compound diet for monkeys. J Hyg (Lond) 1949 Jun;47(2):209–212. doi: 10.1017/s0022172400014480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH S. W., WEISS S. B., KENNEDY E. P. The enzymatic dephosphorylation of phosphatidic acids. J Biol Chem. 1957 Oct;228(2):915–922. [PubMed] [Google Scholar]
- Scharff R., Wool I. G. Accumulation of amino acids in muscle of perfused rat heart. Effect of insulin. Biochem J. 1965 Oct;97(1):257–271. doi: 10.1042/bj0970257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIELAND O., SUYTER M. Glycerokinase; Isolierung und Eigenschaften des Enzyms. Biochem Z. 1957;329(4):320–331. [PubMed] [Google Scholar]
- WINEGRAD A. I., RENOLD A. E. Studies on rat adipose tissue in vitro. I. Effects of insulin on the metabolism of glucose, pyruvate, and acetate. J Biol Chem. 1958 Aug;233(2):267–272. [PubMed] [Google Scholar]
- WOLLENBERGER A., RISTAU O., SCHOFFA G. [A simple technic for extremely rapid freezing of large pieces of tissue]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1960;270:399–412. [PubMed] [Google Scholar]


