Abstract
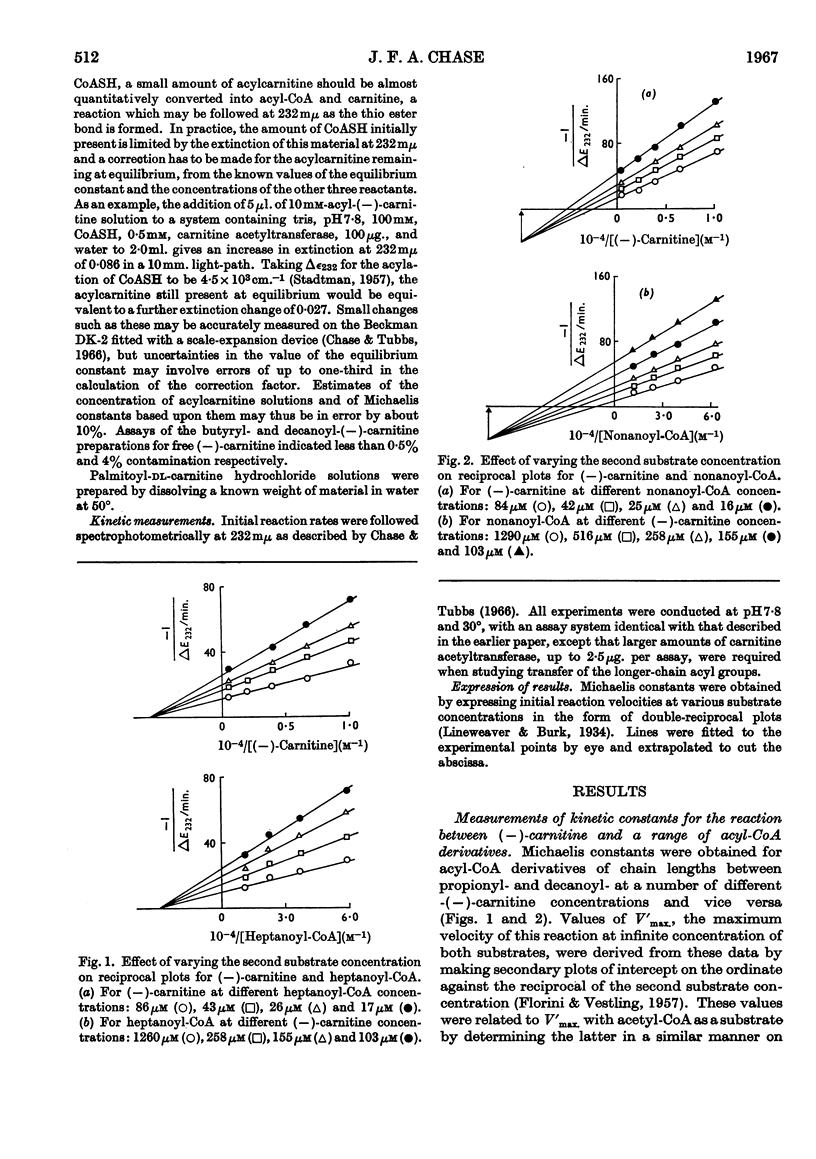
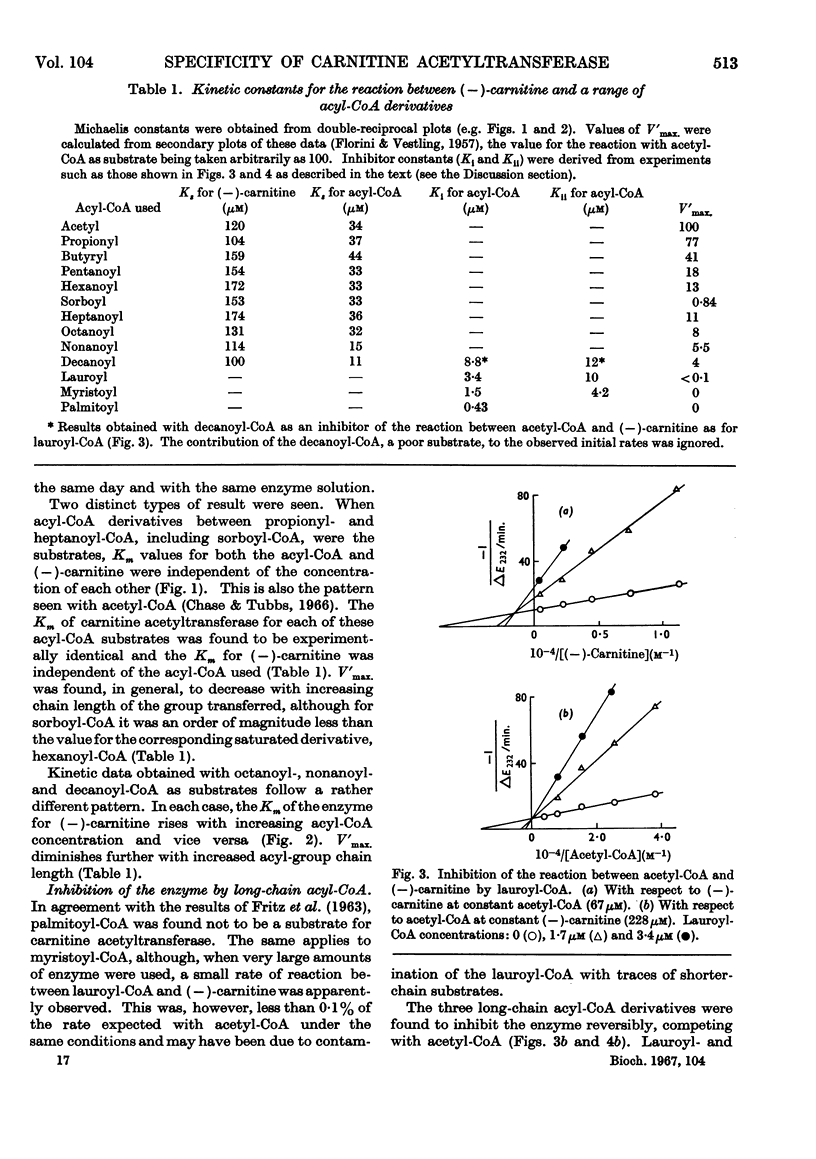
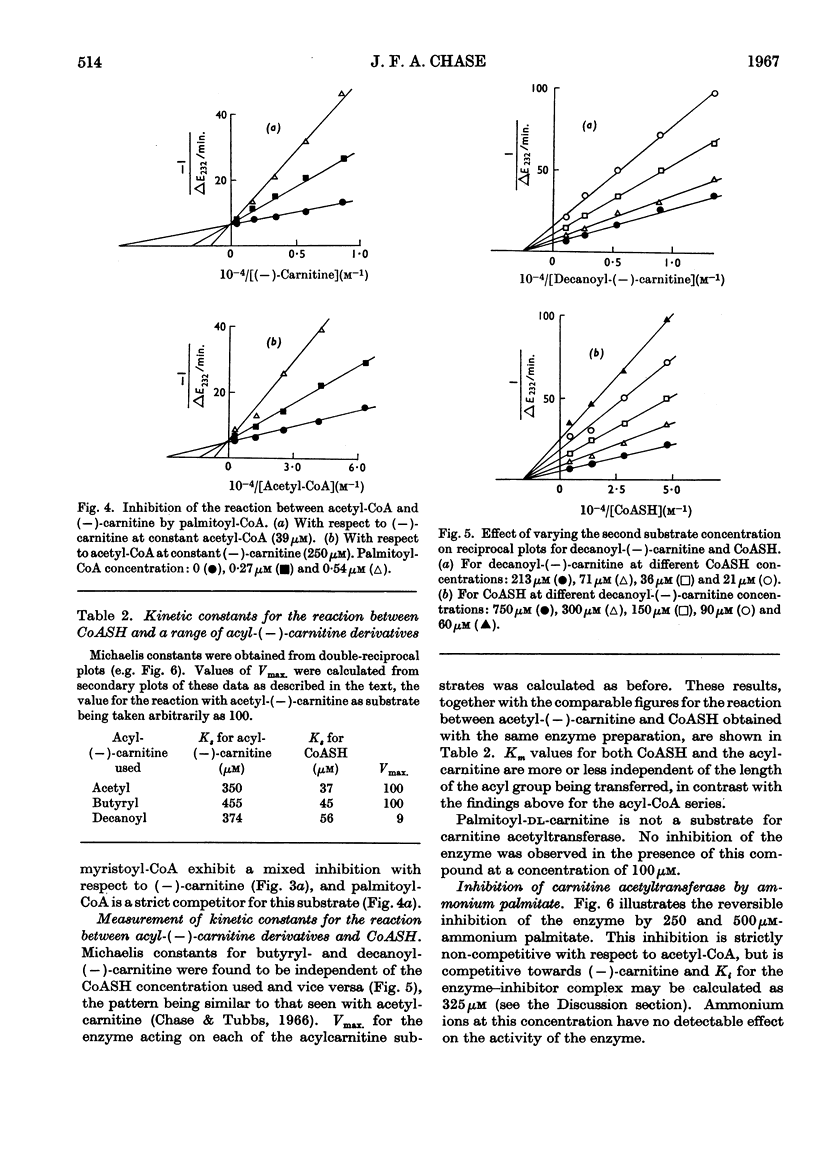
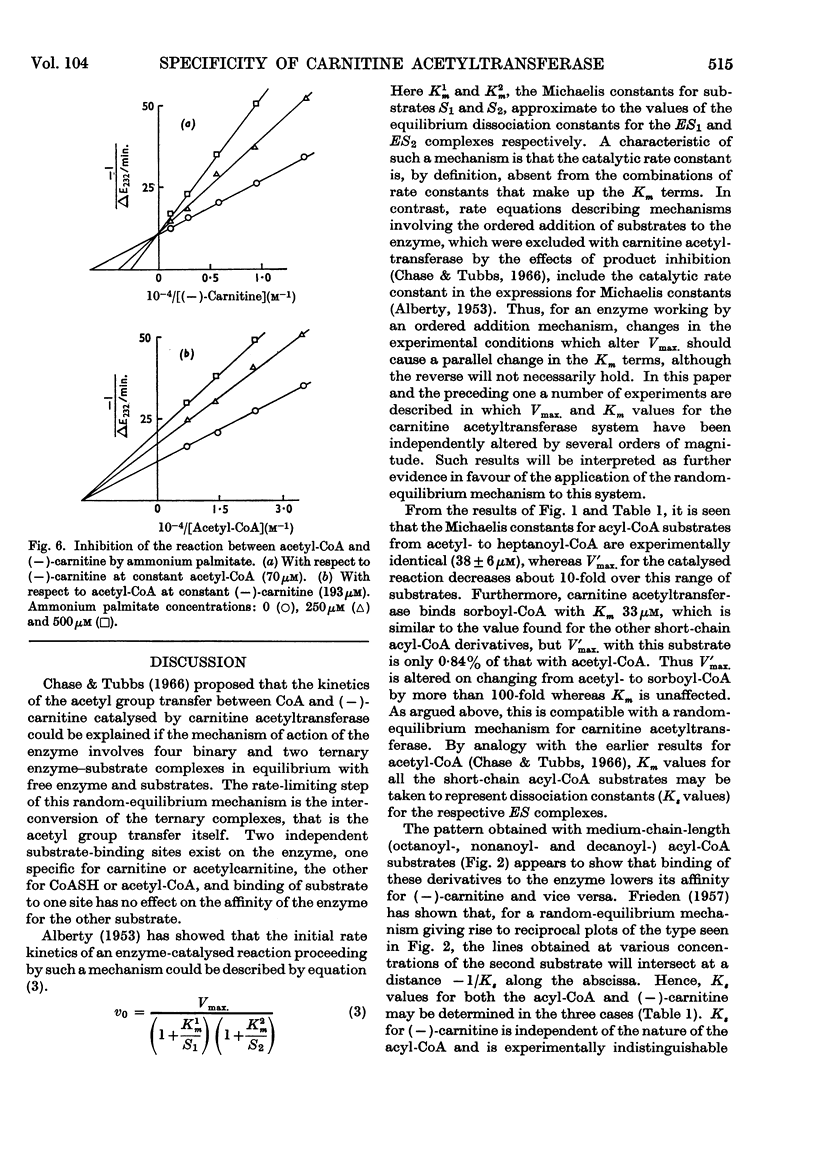
1. A study of the acyl group specificity of the carnitine acetyltransferase reaction [acyl-(−)carnitine+CoASH ⇌ (−)-carnitine+acyl-CoA] has been made with the enzyme from pigeon breast muscle. Acyl groups containing up to 10 carbon atoms are transferred and detailed kinetic investigations with a range of acyl-CoA and acylcarnitine substrates are reported. 2. Acyl-CoA derivatives with 12 or more carbon atoms in the acyl group are potent reversible inhibitors of carnitine acetyltransferase, competing with acetyl-CoA. Lauroyl- and myristoyl-CoA show a mixed inhibition with respect to (−)-carnitine, but palmitoyl-CoA competes strictly with this substrate also. Palmitoyl-dl-carnitine shows none of these effects. 3. Ammonium palmitate inhibits the enzyme competitively with respect to (−)-carnitine and non-competitively with respect to acetyl-CoA. 4. It is suggested that a hydrophobic site exists on the carnitine acetyltransferase molecule. The hydrocarbon chain of an acyl-CoA derivative containing eight or more carbon atoms in the acyl group may interact with this, which results in enhanced acyl-CoA binding. Competition occurs between ligands bound to this hydrophobic site and the carnitine binding site. 5. The possible physiological significance of long-chain acyl-CoA inhibition of this enzyme is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEENAKKERS A. M., KLINGENBERG M. CARNITINE-COENZYME A TRANSACETYLASE IN MITOCHONDRIA FROM VARIOUS ORGANS. Biochim Biophys Acta. 1964 Apr 20;84:205–207. doi: 10.1016/0926-6542(64)90081-2. [DOI] [PubMed] [Google Scholar]
- BREMER J. Carnitine in intermediary metabolism. Reversible acetylation of carnitine by mitochondria. J Biol Chem. 1962 Jul;237:2228–2231. [PubMed] [Google Scholar]
- BREMER J. Carnitine in intermediary metabolism. The metabolism of fatty acid esters of carnitine by mitochondria. J Biol Chem. 1962 Dec;237:3628–3632. [PubMed] [Google Scholar]
- CHASE J. F., PEARSON D. J., TUBBS P. K. THE PREPARATION OF CRYSTALLIN CARNITINE ACETYLTRANSFERASE. Biochim Biophys Acta. 1965 Jan;96:162–165. doi: 10.1016/0005-2787(65)90622-2. [DOI] [PubMed] [Google Scholar]
- Chase J. F., Tubbs P. K. Some kinetic studies on the mechanism of action of carnitine acetyltransferase. Biochem J. 1966 Apr;99(1):32–40. doi: 10.1042/bj0990032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase J. F. pH-dependence of carnitine acetyltransferase activity. Biochem J. 1967 Aug;104(2):503–509. doi: 10.1042/bj1040503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- FLORINI J. R., VESTLING C. S. Graphical determination of the dissociation constants for two-substrate enzyme systems. Biochim Biophys Acta. 1957 Sep;25(3):575–578. doi: 10.1016/0006-3002(57)90529-2. [DOI] [PubMed] [Google Scholar]
- FRAENKEL G., FRIEDMAN S. Carnitine. Vitam Horm. 1957;15:73–118. doi: 10.1016/s0083-6729(08)60508-7. [DOI] [PubMed] [Google Scholar]
- FRITZ I. B., SCHULTZ S. K., SRERE P. A. Properties of partially purified carnitine acetyltransferase. J Biol Chem. 1963 Jul;238:2509–2517. [PubMed] [Google Scholar]
- KORNACKER M. S., LOWENSTEIN J. M. CITRATE AND THE CONVERSION OF CARBOHYDRATE INTO FAT. THE ACTIVITIES OF CITRATE-CLEAVAGE ENZYME AND ACETATE THIOKINASE IN LIVERS OF STARVED AND RE-FED RATS. Biochem J. 1965 Jan;94:209–215. doi: 10.1042/bj0940209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornacker M. S., Ball E. G. Citrate cleavage in adipose tissue. Proc Natl Acad Sci U S A. 1965 Sep;54(3):899–904. doi: 10.1073/pnas.54.3.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynen F., Matsuhashi M., Numa S., Schweizer E. The cellular control of fatty acid synthesis at the enzymatic level. Biochem Soc Symp. 1963;24:43–56. [PubMed] [Google Scholar]
- SRERE P. A., KOSICKI G. W. The purification of citrate-condensing enzyme. J Biol Chem. 1961 Oct;236:2557–2559. [PubMed] [Google Scholar]
- TUBBS P. K. INHIBITION OF CITRATE FORMATION BY LONG-CHAIN ACYL THIOESTERS OF COENZYME A AS A POSSIBLE CONTROL MECHANISM IN FATTY ACID BIOSYNTHESIS. Biochim Biophys Acta. 1963 Oct 22;70:608–609. doi: 10.1016/0006-3002(63)90804-7. [DOI] [PubMed] [Google Scholar]
- Taketa K., Pogell B. M. The effect of palmityl coenzyme A on glucose 6-phosphate dehydrogenase and other enzymes. J Biol Chem. 1966 Feb 10;241(3):720–726. [PubMed] [Google Scholar]
- WIELAND O., WEISS L. INHIBITION OF CITRATE-SYNTHASE BY PALMITYL-COENZYME A. Biochem Biophys Res Commun. 1963 Sep 10;13:26–31. doi: 10.1016/0006-291x(63)90156-6. [DOI] [PubMed] [Google Scholar]


