Abstract
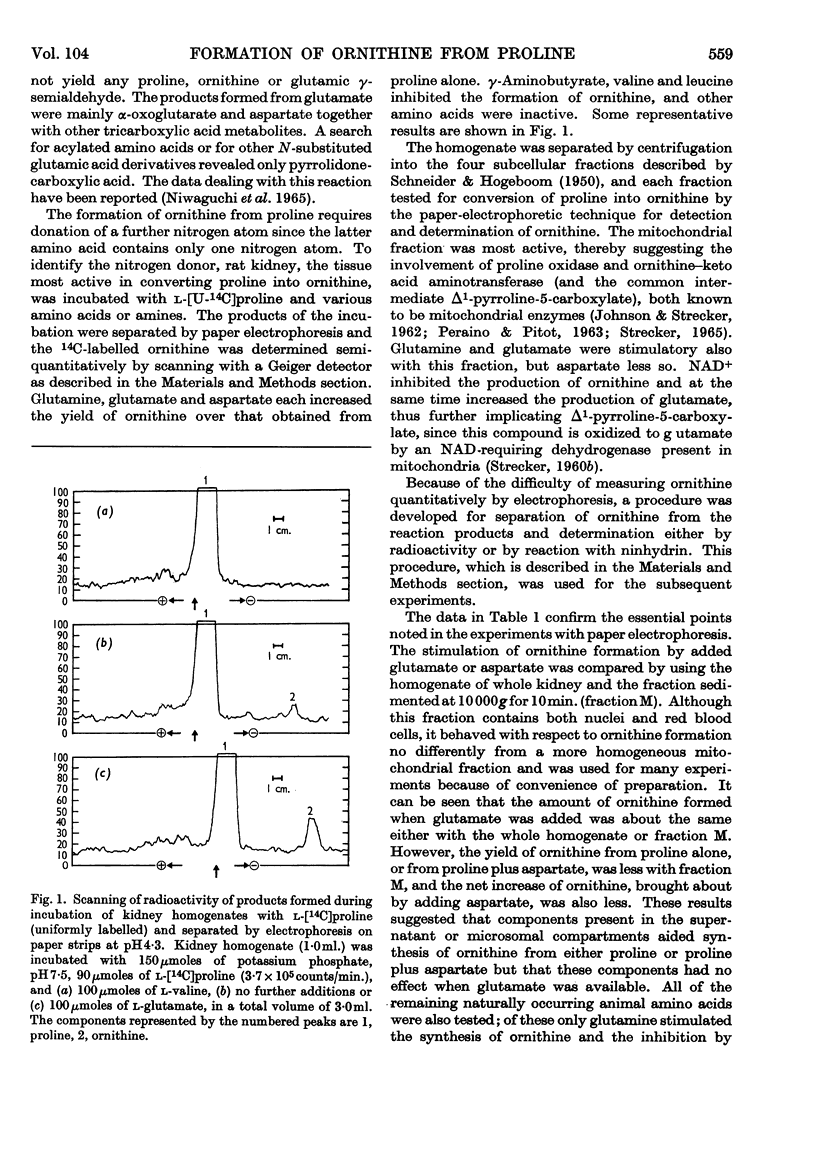
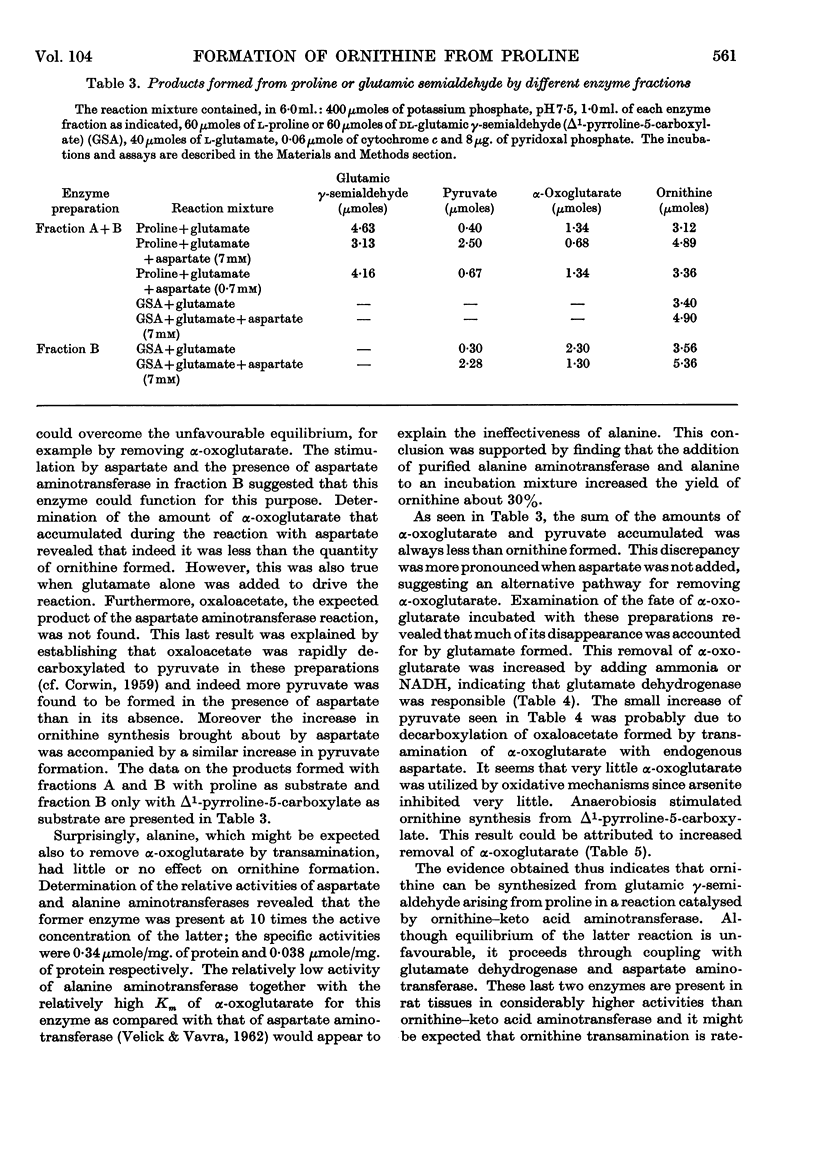
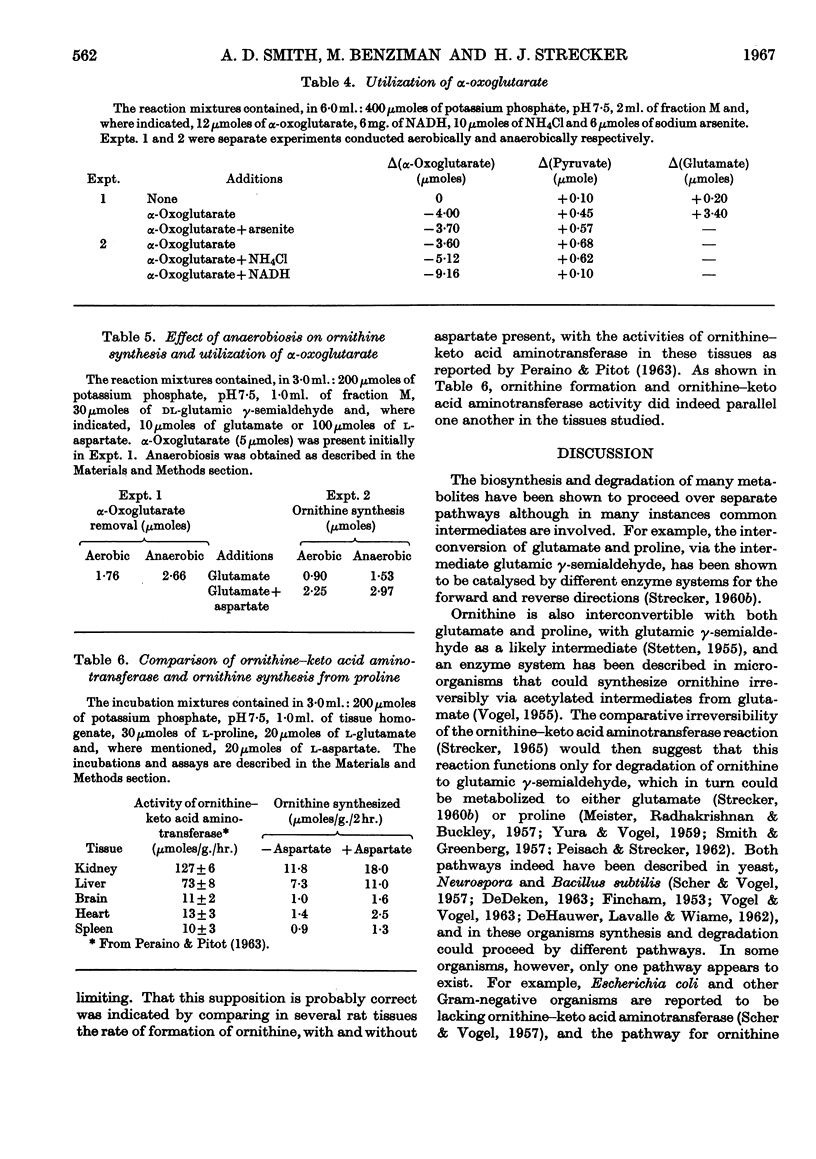
1. Homogenates of liver or kidney from rat, mouse, dog and guinea pig formed ornithine from proline but not from glutamate. Rat kidney was most active in this reaction and was used for further studies. 2. The overall reaction was found to be catalysed by proline oxidase to yield glutamic γ-semialdehyde, followed by transamination of this product with glutamate as catalysed by ornithine–keto acid aminotransferase. 3. The unfavourable equilibrium of the ornithine–keto acid aminotransferase reaction was overcome chiefly by glutamate dehydrogenase in the tissue, which removed the α-oxoglutarate produced, by reduction with endogenous ammonia and NADH. 4. Aspartate aminotransferase in these preparations also aided in the removal of α-oxoglutarate. In this case the overall reaction was driven also by the rapid decarboxylation of oxaloacetate. 5. No evidence could be found for a pathway of ornithine synthesis involving acylated intermediates as has been observed in some micro-organisms. 6. The rate of ornithine synthesis in homogenates of several rat tissues paralleled the activity of ornithine–keto acid aminotransferase in these tissues, indicating that this enzyme was rate-determining for the synthesis. 7. The possible influence of these reactions on urea synthesis is discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABELSON P. H. Amino acid biosynthesis in Escherichia coli: isotopic competition with C14-glucose. J Biol Chem. 1954 Jan;206(1):335–343. [PubMed] [Google Scholar]
- ABELSON P. H., BOLTON E. T., ALDOUS E. Utilization of carbon dioxide in the synthesis of proteins by Escherichia coli. II. J Biol Chem. 1952 Sep;198(1):173–178. [PubMed] [Google Scholar]
- Abelson P. H., Bolton E., Britten R., Cowie D. B., Roberts R. B. Synthesis of the Aspartic and Glutamic Families of Amino Acids in Escherichia Coli. Proc Natl Acad Sci U S A. 1953 Oct;39(10):1020–1026. doi: 10.1073/pnas.39.10.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHINARD F. P. Photometric estimation of proline and ornithine. J Biol Chem. 1952 Nov;199(1):91–95. [PubMed] [Google Scholar]
- CORWIN L. M. Oxaloacetic decarboxylase from rat liver mitochondria. J Biol Chem. 1959 Jun;234(6):1338–1341. [PubMed] [Google Scholar]
- DE HAUWER G., LAVALLE R., WIAME J. M. [Regulation of the oxidation of glutamic semialdehyde in Bacillus subtilis]. Arch Int Physiol Biochim. 1962 Dec;70:746–748. [PubMed] [Google Scholar]
- DEDEKEN R. H. BIOSYNTH'ESE DE L'ARGININE CHEZ LA LEVURE. I. LE SORT DE LA N-ALPHA-AC'ETYLORINITHINE. Biochim Biophys Acta. 1963 Dec 13;78:606–616. doi: 10.1016/0006-3002(63)91026-6. [DOI] [PubMed] [Google Scholar]
- DOUNCE A. L., WITTER R. F., MONTY K. J., PATE S., COTTONE M. A. A method for isolating intact mitochondria and nuclei from the same homogenate, and the influence of mitochondrial destruction on the properties of cell nuclei. J Biophys Biochem Cytol. 1955 Mar;1(2):139–153. doi: 10.1083/jcb.1.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINCHAM J. R. S. Ornithine transaminase in Neurospora and its relation to the biosynthesis of proline. Biochem J. 1953 Jan;53(2):313–320. doi: 10.1042/bj0530313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON A. B., STRECKER H. J. The interconversion of glutamic acid and proline. IV. The oxidation of proline by rat liver mitochondria. J Biol Chem. 1962 Jun;237:1876–1882. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MEISTER A., RADHAKRISHNAN A. N., BUCKLEY S. D. Enzymatic synthesis of L-pipecolic acid and L-proline. J Biol Chem. 1957 Dec;229(2):789–800. [PubMed] [Google Scholar]
- Niwaguchi T., Motohashi N., Strecker H. J. The metabolic conversion of L-glutamate to pyrrolidone carboxylate in rat tissues. Biochem Z. 1965 Aug 19;342(4):469–484. [PubMed] [Google Scholar]
- PEISACH J., STRECKER H. J. The interconversion of glutamic acid and proline. V. The reduction of delta 1-pyrroline-5-carboxylic acid to proline. J Biol Chem. 1962 Jul;237:2255–2260. [PubMed] [Google Scholar]
- RATNER S. Urea synthesis and metabolism of arginine and citrulline. Adv Enzymol Relat Subj Biochem. 1954;15:319–387. doi: 10.1002/9780470122600.ch8. [DOI] [PubMed] [Google Scholar]
- ROSEN H. A modified ninhydrin colorimetric analysis for amino acids. Arch Biochem Biophys. 1957 Mar;67(1):10–15. doi: 10.1016/0003-9861(57)90241-2. [DOI] [PubMed] [Google Scholar]
- Rose W. C., Rice E. E. THE SIGNIFICANCE OF THE AMINO ACIDS IN CANINE NUTRITION. Science. 1939 Aug 25;90(2330):186–187. doi: 10.1126/science.90.2330.186. [DOI] [PubMed] [Google Scholar]
- SMITH M. E., GREENBERG D. M. Preparation and properties of partially purified glutamic semialdehyde reductase. J Biol Chem. 1957 May;226(1):317–327. [PubMed] [Google Scholar]
- STRECKER H. J. PURIFICATION AND PROPERTIES OF RAT LIVER ORNITHINE DELTA-TRANSAMINASE. J Biol Chem. 1965 Mar;240:1225–1230. [PubMed] [Google Scholar]
- STRECKER H. J. The interconversion of glutamic acid and proline. I. The formation of delta1-pyrroline-5-carboxylic acid from glutamic acid in Escherichia coli. J Biol Chem. 1957 Apr;225(2):825–834. [PubMed] [Google Scholar]
- STRECKER H. J. The interconversion of glutamic acid and proline. II. The preparation and properties of delta 1-pyrroline-5-carboxylic acid. J Biol Chem. 1960 Jul;235:2045–2050. [PubMed] [Google Scholar]
- Scher W. I., Vogel H. J. OCCURRENCE OF ORNITHINE delta-TRANSAMINASE: A DICHOTOMY. Proc Natl Acad Sci U S A. 1957 Sep 15;43(9):796–803. doi: 10.1073/pnas.43.9.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL R. H., VOGEL H. J. Acetylated intermediates of arginine synthesis in Bacillus subtilis. Biochim Biophys Acta. 1963 Jan 1;69:174–176. doi: 10.1016/0006-3002(63)91241-1. [DOI] [PubMed] [Google Scholar]
- YURA T., VOGEL H. J. Pyrroline-5-carboxylate reductase of Neurospora crassa; partial purification and some properties. J Biol Chem. 1959 Feb;234(2):335–338. [PubMed] [Google Scholar]


