Abstract
1. Acid proteinase from rabbit liver lysosomes was purified about 1000-fold, on a protein basis. 2. The purification procedure involved isolation of a lysosomal–mitochondrial pellet and conversion of this into an acetone-dried powder. 3. The enzyme was extracted with an acidic buffer and subjected to column chromatography with DEAE-Sephadex and Sephadex G-100. 4. The molecular weight of the enzyme was 50000–52000. 5. Maximal activity against haemoglobin was obtained at pH3·2; serum albumin was attacked, but very much more slowly. 6. Several possible inhibitors of the enzyme were tested. Thiol-blocking reagents, several inhibitors of trypsin and chymotrypsin, and a chelating agent were without effect. 7. The enzyme was competitively inhibited by 3-phenylpyruvic acid at low concentrations. 8. Dithiothreitol caused rapid inactivation of the enzyme at pH8. 9. It is concluded that this enzyme is a form of cathepsin D, which may be widely distributed in lysosomes.
Full text
PDF


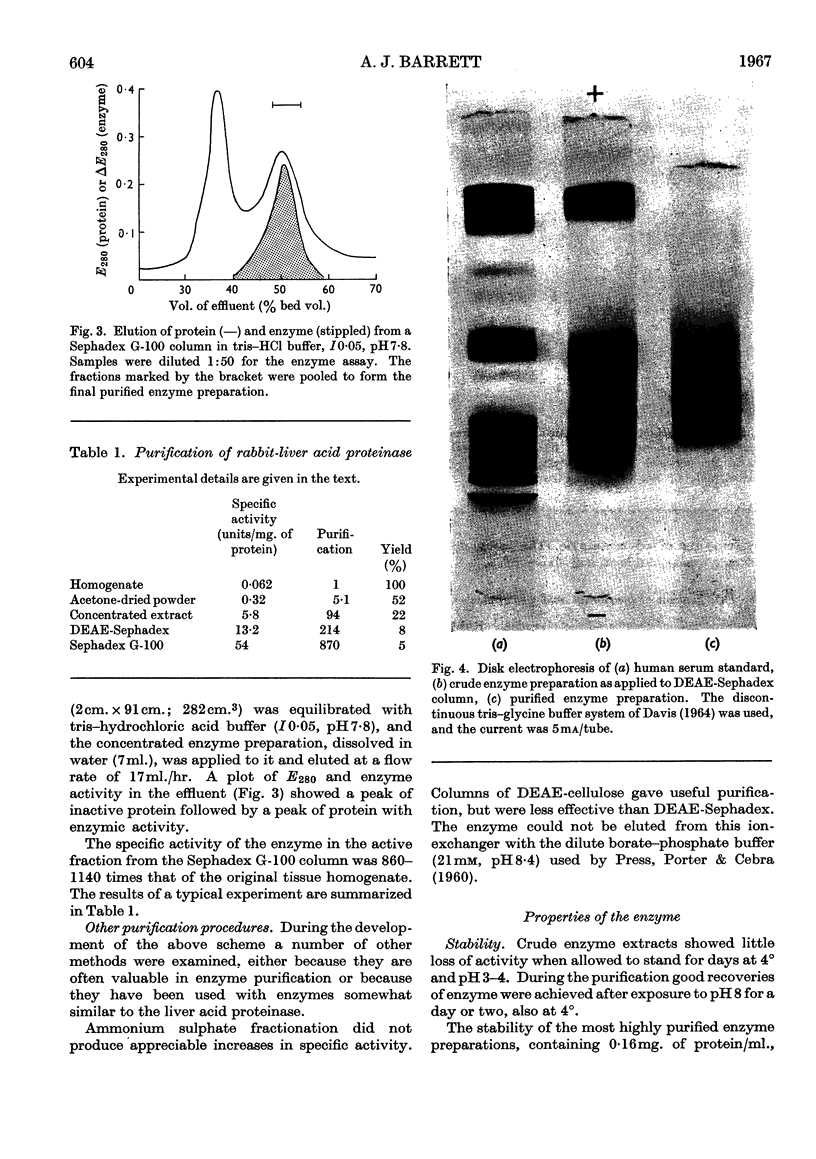
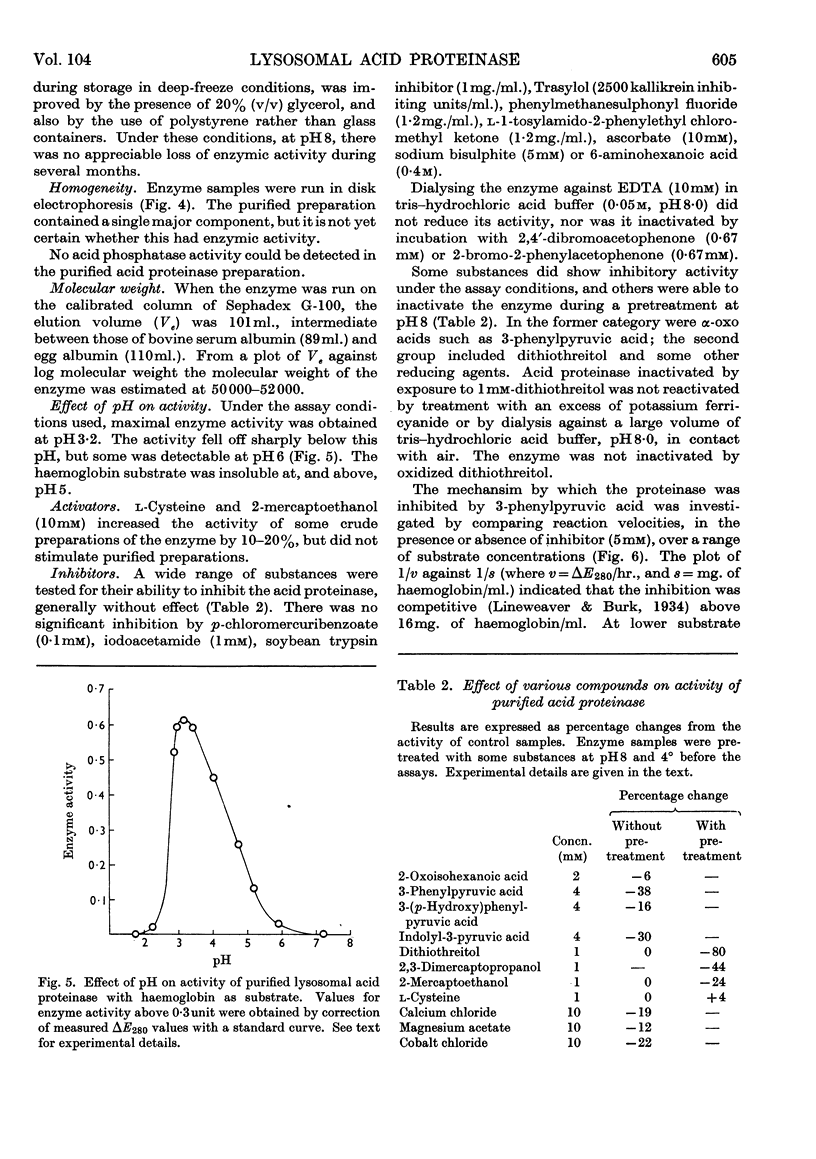
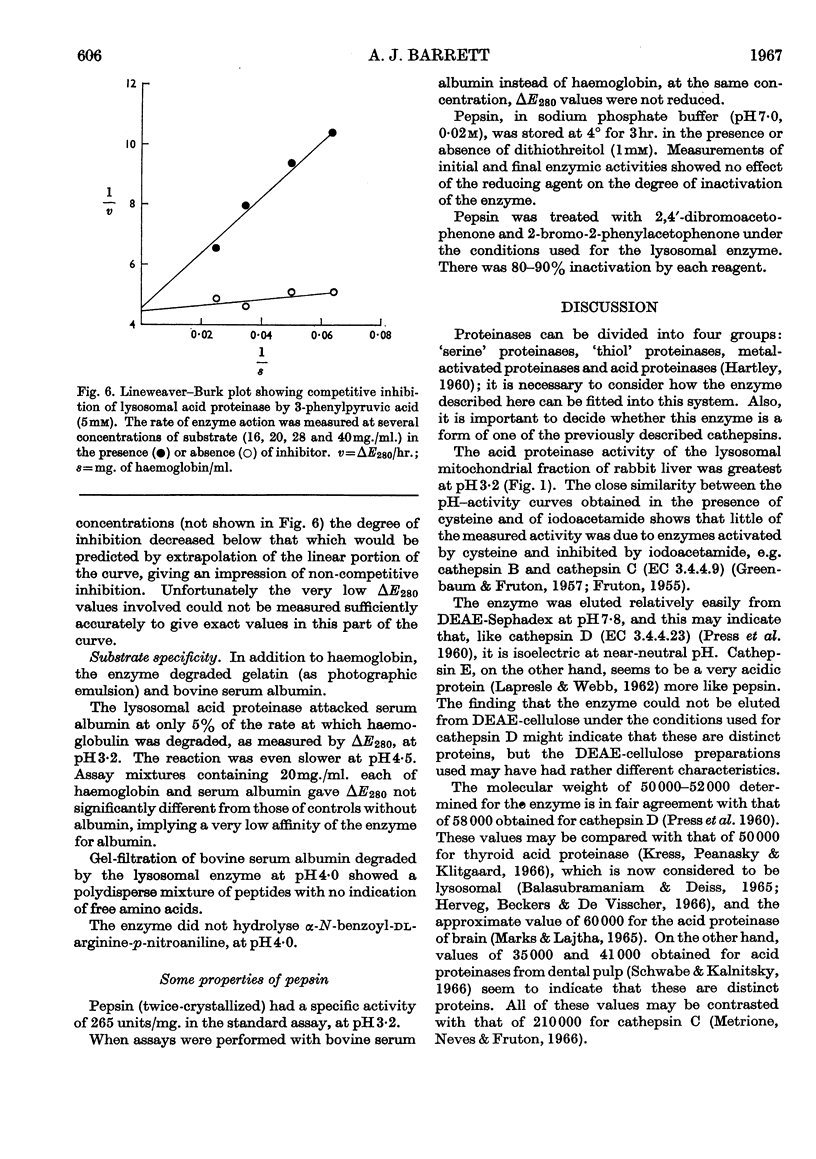


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali S. Y. The degradation of cartilage matrix by an intracellular protease. Biochem J. 1964 Dec;93(3):611–618. doi: 10.1042/bj0930611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLELAND W. W. DITHIOTHREITOL, A NEW PROTECTIVE REAGENT FOR SH GROUPS. Biochemistry. 1964 Apr;3:480–482. doi: 10.1021/bi00892a002. [DOI] [PubMed] [Google Scholar]
- DANNENBERG A. M., Jr, SMITH E. L. Action of proteinase I of bovine lung; hydrolysis of the oxidized B chain of insulin; polymer formation from amino acid esters. J Biol Chem. 1955 Jul;215(1):55–66. [PubMed] [Google Scholar]
- DANNENBERG A. M., Jr, SMITH E. L. Proteolytic enzymes of lung. J Biol Chem. 1955 Jul;215(1):45–54. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DE DUVE C., WATTIAUX R., BAUDHUIN P. Distribution of enzymes between subcellular fractions in animal tissues. Adv Enzymol Relat Subj Biochem. 1962;24:291–358. doi: 10.1002/9780470124888.ch6. [DOI] [PubMed] [Google Scholar]
- DOPHEIDE T. A., TODD P. E. HYDROLYSIS OF THE A AND B CHAINS OF OXIDISED INSULIN BY ADRENAL ACID PROTEINASE. Biochim Biophys Acta. 1964 Apr 4;86:130–135. doi: 10.1016/0304-4165(64)90166-7. [DOI] [PubMed] [Google Scholar]
- ERLANGER B. F., KOKOWSKY N., COHEN W. The preparation and properties of two new chromogenic substrates of trypsin. Arch Biochem Biophys. 1961 Nov;95:271–278. doi: 10.1016/0003-9861(61)90145-x. [DOI] [PubMed] [Google Scholar]
- ERLANGER B. F., VRATSANOS S. M., WASSERMANN N., COOPER A. G. SPECIFIC AND REVERSIBLE INACTIVATION OF PEPSIN. J Biol Chem. 1965 Aug;240:PC3447–PC3448. [PubMed] [Google Scholar]
- Erlanger B. F., Vratsanos S. M., Wassermann N., Cooper A. G. A chemical investigation of the active center of pepsin. Biochem Biophys Res Commun. 1966 May 3;23(3):243–245. doi: 10.1016/0006-291x(66)90535-3. [DOI] [PubMed] [Google Scholar]
- FELL H. B., DINGLE J. T. Studies on the mode of action of excess of vitamin A. 6. Lysosomal protease and the degradation of cartilage matrix. Biochem J. 1963 May;87:403–408. doi: 10.1042/bj0870403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENBAUM L. M., FRUTON J. S. Purification and properties of beef spleen cathepsin B. J Biol Chem. 1957 May;226(1):173–180. [PubMed] [Google Scholar]
- Geratz J. D. Alpha-keto analogues of amino acids as inhibitors of alpha-chymotrypsin, carboxypeptidase A, and pepsin. Arch Biochem Biophys. 1965 Jul;111(1):134–141. doi: 10.1016/0003-9861(65)90331-0. [DOI] [PubMed] [Google Scholar]
- Greenbaum L. M., Yamafuji K. The in vitro inactivation and formation of plasma kinins by spleen cathepsins. Br J Pharmacol Chemother. 1966 May;27(1):230–238. doi: 10.1111/j.1476-5381.1966.tb01658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARTLEY B. S. Proteolytic enzymes. Annu Rev Biochem. 1960;29:45–72. doi: 10.1146/annurev.bi.29.070160.000401. [DOI] [PubMed] [Google Scholar]
- Herveg J. P., Beckers C., De Visscher M. Lysosomal hydrolases in calf thyroid. Biochem J. 1966 Aug;100(2):540–547. doi: 10.1042/bj1000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolles B., Harrison R. G. Enzymic processes and vascular changes in the skin radiation reaction. Br J Radiol. 1966 Jan;39(457):12–18. doi: 10.1259/0007-1285-39-457-12. [DOI] [PubMed] [Google Scholar]
- Kress L. F., Peanasky R. J., Klitgaard H. M. Purification, properties, and specificity of hog thyroid proteinase. Biochim Biophys Acta. 1966 Feb 14;113(2):375–389. doi: 10.1016/s0926-6593(66)80076-0. [DOI] [PubMed] [Google Scholar]
- LAPRESLE C., WEBB T. Study of a proteolytic enzyme from rabbit spleen. Biochem J. 1960 Sep;76:538–543. doi: 10.1042/bj0760538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAPRESLE C., WEBB T. The purification and properties of a proteolytic enzyme, rabbit cathepsin E, and further studies on rabbit cathepsin D. Biochem J. 1962 Sep;84:455–462. doi: 10.1042/bj0840455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marks N., Lajtha A. Separation of acid and neutral proteinases of brain. Biochem J. 1965 Oct;97(1):74–83. doi: 10.1042/bj0970074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metroione R. M., Neves A. G., Fruton J. S. Purification and properties of dipeptidyl transferase (Cathepsin C). Biochemistry. 1966 May;5(5):1597–1604. doi: 10.1021/bi00869a021. [DOI] [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- PRESS E. M., PORTER R. R., CEBRA J. The isolation and properties of a proteolytic enzyme, cathepsin D, from bovine spleen. Biochem J. 1960 Mar;74:501–514. doi: 10.1042/bj0740501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHOELLMANN G., SHAW E. Direct evidence for the presence of histidine in the active center of chymotrypsin. Biochemistry. 1963 Mar-Apr;2:252–255. doi: 10.1021/bi00902a008. [DOI] [PubMed] [Google Scholar]
- Schwabe C., Kalnitsky G. A peptidohydrolase from mammalian fibroblasts (bovine dental pulp). Biochemistry. 1966 Jan;5(1):158–168. doi: 10.1021/bi00865a021. [DOI] [PubMed] [Google Scholar]
- TORRIANI A. Influence of inorganic phosphate in the formation of phosphatases by Escherichia coli. Biochim Biophys Acta. 1960 Mar 11;38:460–469. doi: 10.1016/0006-3002(60)91281-6. [DOI] [PubMed] [Google Scholar]
- Wasi S., Uriuhara T., Taichman N. S., Murray R. K., Movat H. Z. Proteolytic activity in the serum of rabbits during anaphylaxis. Experientia. 1966 Mar 15;22(3):196–198. doi: 10.1007/BF01897735. [DOI] [PubMed] [Google Scholar]
- Weinstock I. M. Comparative biochemistry of myopathies. Ann N Y Acad Sci. 1966 Sep 9;138(1):199–212. [PubMed] [Google Scholar]
- Woessner J. F., Jr Acid hydrolases of the rat uterus in relation to pregnancy, post-partum involution and collagen breakdown. Biochem J. 1965 Dec;97(3):855–866. doi: 10.1042/bj0970855. [DOI] [PMC free article] [PubMed] [Google Scholar]



