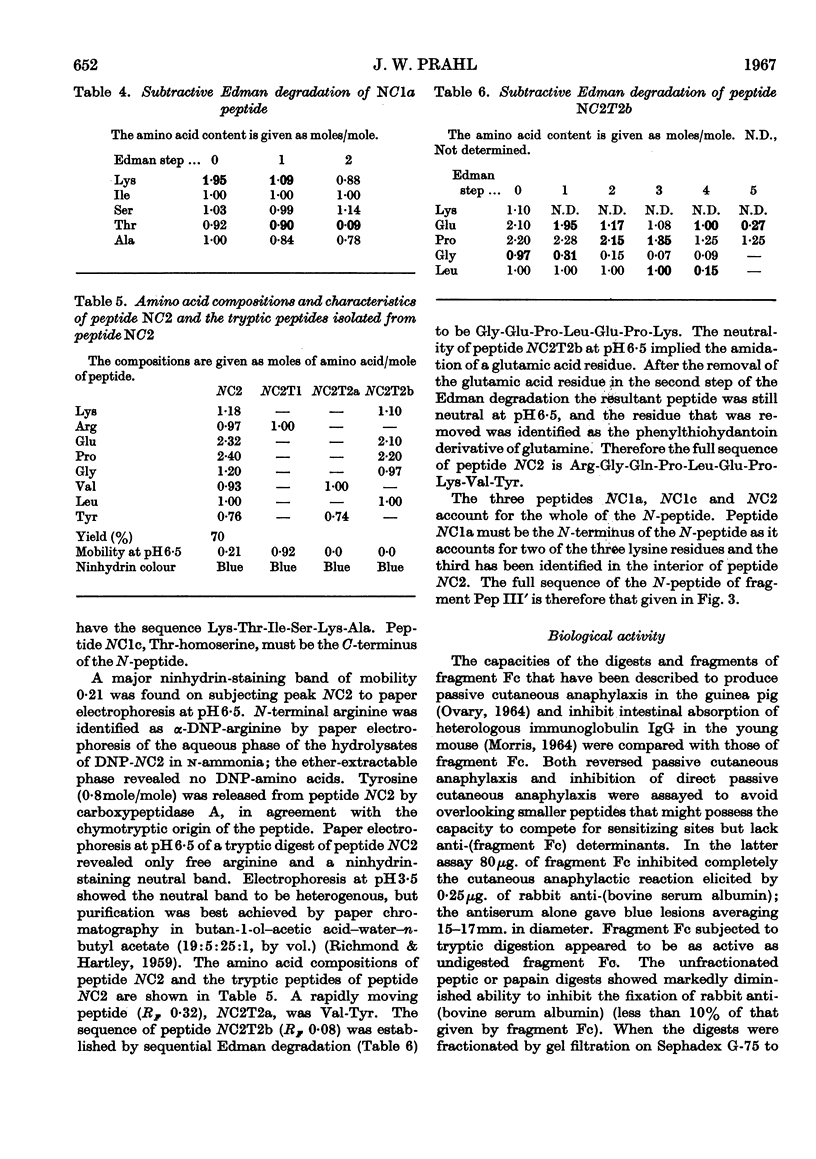
Abstract
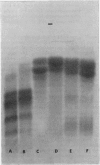
The digestion of the Fc fragment of rabbit immunoglobulin IgG by several proteolytic enzymes was investigated by using gel filtration and starch-gel electrophoresis in 8m-urea–formate as criteria of the extent of degradation. Though fragment Fc and mildly reduced fragment Fc proved resistant to tryptic hydrolysis, papain and pepsin cleaved the fragment at acidic pH values and appeared to give rise to a similar spectrum of products. A (limit) peptide comprising the C-terminal 113 residues of the heavy chain was isolated and identified from the pepsin-digest products of fragment Fc. The products of proteolytic digestion of fragment Fc were no longer able to inhibit passive cutaneous anaphylaxis by rabbit anti-(bovine serum albumin) or demonstrate reversed passive cutaneous anaphylaxis in the guinea pig. Nor were they able to inhibit the intestinal absorption of heterologous immunoglobulin IgG in the young mouse. These studies imply that the site or sites responsible for these biological properties of intact fragment Fc reside in the N-terminal 30–40% of the fragment.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRADBURY J. H. The kinetics of hydrazinolysis of simple peptides in anhydrous hydrazine. Biochem J. 1958 Mar;68(3):475–482. doi: 10.1042/bj0680475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAMBELL F. W., HEMMINGS W. A., OAKLEY C. L., PORTER R. R. The relative transmission of the fractions of papain hydrolyzed homologous gamma-globulin from the uterine cavity to the foetal circulation in the rabbit. Proc R Soc Lond B Biol Sci. 1960 Mar 1;151:478–482. doi: 10.1098/rspb.1960.0011. [DOI] [PubMed] [Google Scholar]
- Berken A., Benacerraf B. Properties of antibodies cytophilic for macrophages. J Exp Med. 1966 Jan 1;123(1):119–144. doi: 10.1084/jem.123.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CESSI C. Micrometodo per la determinazione della glucosamina in liquidi biologici. Boll Soc Ital Biol Sper. 1952 Apr;28(4):858–859. [PubMed] [Google Scholar]
- FLEISCHMAN J. B., PAIN R. H., PORTER R. R. Reduction of gamma-globulins. Arch Biochem Biophys. 1962 Sep;Suppl 1:174–180. [PubMed] [Google Scholar]
- GOODMAN J. W. IMMUNOLOGICALLY ACTIVE FRAGMENTS OF RABBIT GAMMA GLOBULIN. Biochemistry. 1964 Jun;3:857–863. doi: 10.1021/bi00894a023. [DOI] [PubMed] [Google Scholar]
- Hill R. L., Delaney R., Lebovitz H. E., Fellows R. E., Jr Studies on the amino acid sequence of heavy chains from rabbit immunoglobulin G. Proc R Soc Lond B Biol Sci. 1966 Nov 22;166(1003):159–175. doi: 10.1098/rspb.1966.0091. [DOI] [PubMed] [Google Scholar]
- Inman F. P., Nisonoff A. Reversible dissociation of fragment Fc of rabbit gamma-G-immunoglobulin. J Biol Chem. 1966 Jan 25;241(2):322–329. [PubMed] [Google Scholar]
- KIMMEL J. R., SMITH E. L. Crystalline papain. I. Preparation, specificity, and activation. J Biol Chem. 1954 Apr;207(2):515–531. [PubMed] [Google Scholar]
- KONIGSBERG W., HILL R. J. The structure of human hemoglobin. III. The sequence of amino acids in the tryptic peptides of the alpha chain. J Biol Chem. 1962 Aug;237:2547–2561. [PubMed] [Google Scholar]
- KOSTKA V., CARPENTER F. H. INHIBITION OF CHYMOTRYPSIN ACTIVITY IN CRYSTALLINE TRYPSIN PREPARATIONS. J Biol Chem. 1964 Jun;239:1799–1803. [PubMed] [Google Scholar]
- Kekwick R. A. The serum proteins in multiple myelomatosis. Biochem J. 1940 Sep;34(8-9):1248–1257. doi: 10.1042/bj0341248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORRIS I. G. THE TRANSMISSION OF ANTIBODIES AND NORMAL GAMMA-GLOBULINS ACROSS THE YOUNG MOUSE GUT. Proc R Soc Lond B Biol Sci. 1964 May 19;160:276–292. doi: 10.1098/rspb.1964.0040. [DOI] [PubMed] [Google Scholar]
- MURACHI T., NEURATH H. Fractionation and specificity studies on stem bromelain. J Biol Chem. 1960 Jan;235:99–107. [PubMed] [Google Scholar]
- NELSON C. A. ISOLATION OF A NEW INTERMEDIATE IN THE PAPAIN CLEAVAGE OF RABBIT GAMMA-GLOBULIN. J Biol Chem. 1964 Nov;239:3727–3732. [PubMed] [Google Scholar]
- NISONOFF A., WISSLER F. C., LIPMAN L. N., WOERNLEY D. L. Separation of univalent fragments from the bivalent rabbit antibody molecule by reduction of disulfide bonds. Arch Biochem Biophys. 1960 Aug;89:230–244. doi: 10.1016/0003-9861(60)90049-7. [DOI] [PubMed] [Google Scholar]
- OVARY Z., KARUSH F. Studies on the immunologic mechanism of anaphylaxis. II. Sensitizing and combining capacity in vivo of fractions separated from papain digests of antihapten antibody. J Immunol. 1961 Feb;86:146–150. [PubMed] [Google Scholar]
- PORTER R. R. The fractionation of rabbit gamma-globulin by partition chromatography. Biochem J. 1955 Mar;59(3):405–410. doi: 10.1042/bj0590405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTER R. R. The hydrolysis of rabbit y-globulin and antibodies with crystalline papain. Biochem J. 1959 Sep;73:119–126. doi: 10.1042/bj0730119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POTTS J. T., Jr, YOUNG D. M., ANFINSEN C. B., SANDOVAL A. STUDIES ON RIBONUCLEASE S. I. LIMITED CARBOXYPEPTIDASE DEGRADATION OF RIBONUCLEASE S-PROTEIN AND RIBONUCLEASE S-PEPTIDE: EFFECTS OF CHANGES IN PRIMARY STRUCTURE ON ENZYMIC ACTIVITY. J Biol Chem. 1964 Nov;239:3781–3786. [PubMed] [Google Scholar]
- Paraskevas F., Goodman J. W. Components of fraction 3 and pepsin-digested fraction 3 from papain-digested rabbit gamma-globulin. Immunochemistry. 1965 Dec;2(4):391–399. doi: 10.1016/0019-2791(65)90038-8. [DOI] [PubMed] [Google Scholar]
- Press E. M., Piggot P. J., Porter R. R. The N- and c-terminal amino acid sequences of the heavy chain from a pathological human immunoglobulin IgG. Biochem J. 1966 May;99(2):356–366. doi: 10.1042/bj0990356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RYLE A. P., PORTER R. R. Parapepsins: two proteolytic enzymes associated with porcine pepsin. Biochem J. 1959 Sep;73:75–86. doi: 10.1042/bj0730075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILMAN H. I., CEBRA J. J., GIVOL D. The carboxyl terminal amino acids of rabbit gamma-globulin. J Biol Chem. 1962 Jul;237:2196–2200. [PubMed] [Google Scholar]
- SPIEGELBERG H. L., WEIGLE W. O. THE CATABOLISM OF HOMOLOGOUS AND HETEROLOGOUS 7S GAMMA GLOBULIN FRAGMENTS. J Exp Med. 1965 Mar 1;121:323–338. doi: 10.1084/jem.121.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TARANTA A., FRANKLIN E. C. Complement fixation by antibody fragments. Science. 1961 Dec 15;134(3494):1981–1982. doi: 10.1126/science.134.3494.1981. [DOI] [PubMed] [Google Scholar]
- WEBER G., YOUNG L. B. FRAGMENTATION OF BOVINE SERUM ALBUMIN BY PEPSIN. I. THE ORIGIN OF THE ACID EXPANSION OF THE ALBUMIN MOLECULE. J Biol Chem. 1964 May;239:1415–1423. [PubMed] [Google Scholar]