Abstract
Disk diffusion and broth microdilution susceptibility tests were performed with cefotaxime, ceftriaxone, telithromycin, and erythromycin (control) against 407 selected isolates of Streptococcus pneumoniae. Scattergrams were prepared from the results of these tests, and the current NCCLS guidelines for setting disk diffusion test interpretive criteria were applied. Erythromycin zone diameter breakpoints were confirmed. Telithromycin interpretive criteria for the disk test could be easily set with acceptable discrepancy rates. For cefotaxime and ceftriaxone, the minor discrepancy rates for MICs in the intermediate category ± 1 dilution were far in excess of the acceptable 40% limit, i.e., 52 and 71%, respectively. We conclude that the 30-μg disk of these two drugs cannot be reliably used to test pneumococci.
Susceptibility test interpretive criteria are determined by a number of considerations. For MIC susceptible and resistant breakpoints, these considerations include the pharmacokinetic and/or pharmacodynamic information of the drug in question, correlation of MICs with clinical outcomes, and the drug's MIC distribution for the species for which the drug is indicated.
The susceptible and resistant breakpoints for the disk diffusion test are determined largely by correlating the disk diffusion inhibitory zone diameters with MICs. In the past this has often been accomplished by regression analysis. The ability of regression analysis to suggest appropriate zone diameter breakpoints is dependent upon a fairly even distribution of organisms at each MIC tested, particularly in the range of the intermediate MIC ± 2 to 3 twofold dilutions. With many of the newer antibiotics, however, resistant organisms are rare, and the MIC distribution is heavily weighted toward very susceptible MICs, resulting in an unreliable regression line. To overcome this problem the error rate-bounded method of Metzler and DeHaan (4) as modified by Brunden et al. (2) is commonly used to select disk diffusion test breakpoints, and this method has been accepted by the National Committee for Clinical Laboratory Standards (NCCLS) (5, 6).
In its first approved guidelines (M23-A), the NCCLS indicated that very major discrepancies (resistant MIC and susceptible zone diameter) should be less than 1.5%, and major discrepancies (susceptible MIC and resistant zone diameter) should be less than 3%, using the total population as the denominator (5). No limits on minor discrepancies (intermediate by one method and resistant or susceptible by the other) were provided. Because these discrepancy rates are greatly influenced by the MIC distribution, it was also pointed out that it was more meaningful to calculate the very major and major discrepancy rates with the MIC-resistant and MIC-susceptible populations, respectively, as denominators. However, no limits for discrepancy rates calculated in this manner were provided.
In the second edition of the NCCLS guidelines (M23-A2), it was recognized that because of the ±1 dilution variation of MICs and the ± 3- to 4-mm variation of zone diameters that are intrinsic to the testing systems, it was appropriate to allow greater discrepancy rates for strains with MICs at the intermediate MIC ± 1 twofold concentration (I + 1 to I − 1) and more limited discrepancy rates for strains with MICs with ≥2 twofold concentrations above (≥I + 2) or below (≤I − 2) the intermediate MIC (6). For setting zone diameter criteria with a challenge set of organisms, acceptable discrepancy rates for these three MIC groups have been proposed as follows: for I + 1 to I − 1, <10% major, <10% very major, and <40% minor discrepancies; for ≥I + 2, <2% very major and <5% minor discrepancies; and for ≤I − 2, <2% major and <5% minor discrepancies. For assessing already established criteria with routine clinical isolates, <1.5% very major and <3% major discrepancies using the total population as the denominator was maintained.
In a recent study we tested the susceptibility of a challenge set of Streptococcus pneumoniae isolates to cefotaxime, ceftriaxone, and the ketolide telithromycin by both broth microdilution and disk diffusion methods. These three drugs currently have no accepted disk diffusion interpretive criteria. The purpose of this study is to assess the applicability of the latest NCCLS guidelines for setting disk diffusion breakpoints for these three drugs when S. pneumoniae is tested.
MATERIALS AND METHODS
Microorganisms.
A total of 407 clinical isolates of S. pneumoniae from North America and Europe was selected to give a broad range of MICs with a large proportion of isolates with cefotaxime and ceftriaxone MICs of ≥1.0 μg/ml. Isolates were identified by the laboratories submitting them by their own standard methods and were confirmed in our laboratory by colonial-morphology, optochin susceptibility, and bile solubility tests. Of the 407 isolates, 147 strains exhibited the MLS phenotype and 109 had the M phenotype.
Antimicrobial agents.
The control drug erythromycin, as well as cefotaxime and ceftriaxone, was supplied by PML Microbiologicals, Wilsonville, Oreg. Telithromycin was provided by Aventis Pharmaceuticals, Paris, France. For disk diffusion tests, commercially prepared disks (BBL, Cockeysville, Md.) were used as follows: cefotaxime and ceftriaxone, 30 μg; and erythromycin and telithromycin, 15 μg.
Susceptibility tests.
MICs for each drug were determined by the broth microdilution method described by the NCCLS (7). Serial twofold concentrations of each drug were prepared in cation-adjusted Mueller-Hinton broth supplemented with 3% lysed horse blood. The concentrations tested are indicated in Fig. 1 to 4. Disk diffusion tests were performed as recommended by the NCCLS (8) on Mueller-Hinton agar supplemented with 5% defibrinated sheep blood.
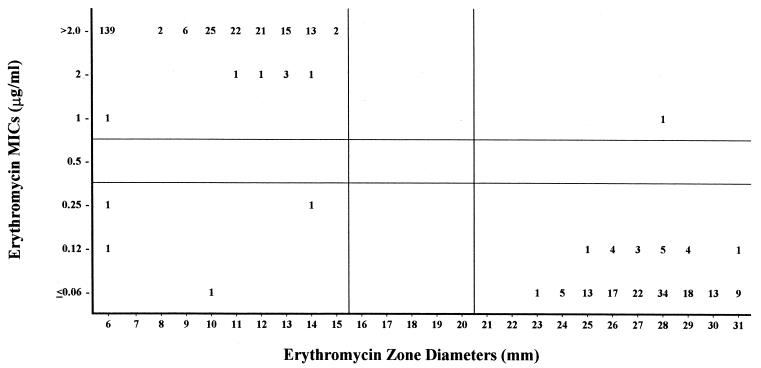
FIG. 1.
Erythromycin MICs versus zone diameters with 407 S. pneumoniae isolates. Horizontal and vertical lines represent established MIC and zone diameter breakpoints, respectively (8).
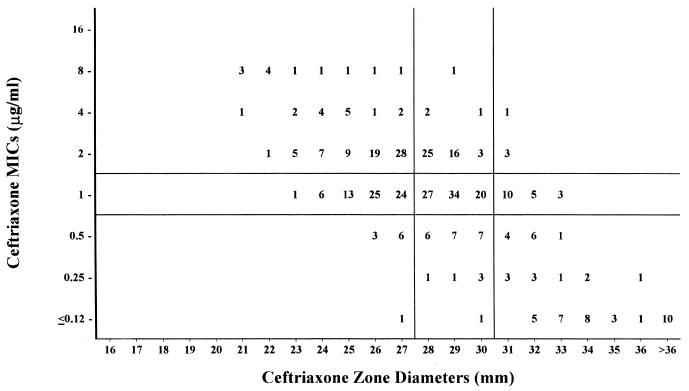
FIG. 4.
Ceftriaxone MICs versus zone diameters with 407 S. pneumoniae isolates. Horizontal and vertical lines represent established MIC breakpoints (8) and best-fit zone diameter breakpoints, respectively.
Quality control.
The quality control strain, S. pneumoniae ATCC 49619 was tested by both methods on each day of testing. All results for erythromycin, cefotaxime, and ceftriaxone as determined by both methods fell within the current NCCLS quality control limits (9).
RESULTS AND DISCUSSION
MIC zone diameter scattergrams for each of the four drugs tested are provided in Fig.1 to 4, and discrepancy rates are listed in Table 1.
TABLE 1.
MIC zone diameter discrepancy rates for four antibiotics with 407 S. pneumoniae isolates
| Antimicrobial agent and MIC range | No. | No. of discrepancies (discrepancy rate [%])a
|
||
|---|---|---|---|---|
| Very major | Major | Minor | ||
| Erythromycin | ||||
| ≥I + 2 | 251 | 0 | NA | 0 |
| I + 1 to I − 1 | 4 | 1 (25) | 2 (50) | 0 |
| ≤I − 2 | 152 | NA | 2 (1.3) | 0 |
| Total | 407 | 1 (0.3) | 4 (1.0) | 0 |
| Telithromycin | ||||
| ≥I + 2 | 3 | 0 | NA | 0 |
| I + 1 to I − 1 | 24 | 0 | 0 | 5 (20.8) |
| ≤I − 2 | 380 | NA | 1 (0.3) | 0 |
| Total | 407 | 0 | 1 (1.3) | 5 (1.2) |
| Cefotaxime | ||||
| ≥I + 2 | 32 | 0 | NA | 4 (12.5) |
| I + 1 to I − 1 | 320 | 6 (1.9) | 9 (2.8) | 135 (51.6) |
| ≤I − 2 | 55 | NA | 0 | 5 (9.1) |
| Total | 407 | 6 (1.5) | 9 (2.2) | 174 (42.8) |
| Ceftriaxone | ||||
| ≥I + 2 | 32 | 1 (3.1) | NA | 4 (12.5) |
| I + 1 to I − 1 | 324 | 3 (0.9) | 9 (2.8) | 231 (71.3) |
| ≤I − 2 | 51 | NA | 1 (2.0) | 6 (11.8) |
| Total | 407 | 4 (1.0) | 10 (2.5) | 241 (59.2) |
NA, not applicable.
Erythromycin.
Erythromycin MICs had a striking bimodal distribution (Fig. 1),and this correlated with a corresponding bimodal distribution of zone diameters. With the current erythromycin MIC and zone diameter breakpoints (3, 9), there was one (0.3%) very major discrepancy, four (1.0%) major discrepancies, and no minor discrepancies. We concluded that the established interpretive criteria for erythromycin are satisfactory. The newer criteria were also used to calculate discrepancy rates. The rates for ≥I + 2 and ≤I − 2 would be acceptable (Table 1), but for the four MICs in I + 1 to I − 1, there was one very major (25%) and two major (50%) discrepancies. There were too few strains in this category to calculate meaningful discrepancy rates. Had these two major and one very major discrepant tests been repeated, as is recommended, it is conceivable that the discrepancies would have vanished.
Telithromycin.
MIC interpretive criteria have yet to be established for telithromycin. For purposes of this discussion, we elected to use the breakpoints proposed by the manufacturer: ≤1.0 μg/ml for susceptible, 2.0 μg/ml for intermediate, and ≥4.0 μg/ml for resistant. Based on these MIC breakpoints, 98% of our isolates would be considered susceptible (Fig. 2).This illustrates the type of results commonly seen with new antibiotics today. With the zone diameter breakpoints selected there was one (0.3%) major discrepancy in the ≤I − 2 group and five (20.8%) minor discrepancies in the I + 1 to I − 1 group, which would be considered acceptable discrepancy rates.
FIG. 2.
Telithromycin MICs versus zone diameters with 407 S. pneumoniae isolates. Horizontal and vertical lines represent proposed MIC breakpoints and corresponding zone diameter breakpoints, respectively.
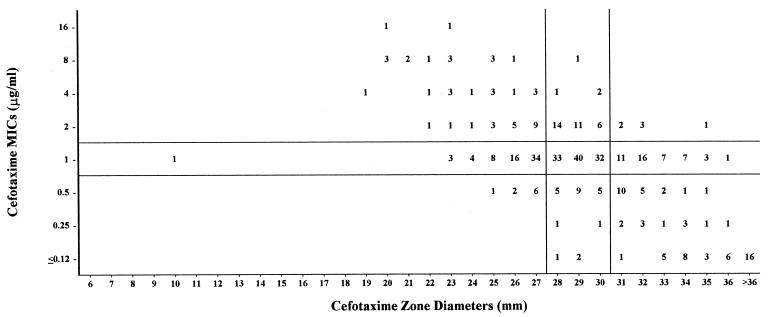
Cefotaxime.
Although MIC breakpoints for cefotaxime have been established for pneumococci (8), attempts to establish zone diameter breakpoints have not been successful (1, 3). The scattergram in Fig. 3illustrates why this is so. The major and very major discrepancy rates fall within acceptable limits, but the minor discrepancy rate is 51.6% in the I + 1 to I − 1 group and is thus well above the 40% limit for this group. Because of the process for selecting these isolates for this study, nearly 80% of isolates fell in the I + 1 to I − 1 group. In a previous study, in which the cefotaxime MICs for the pneumococcal isolates were more representative of what is usually encountered in clinical practice, i.e., only 22% of MICs were in the I + 1 to I − 1 group (1). Nevertheless, >40% of those zone diameters showed minor discrepancies. The latter authors also tested a 1-μg cefotaxime disk, and the minor discrepancy rate for the I + 1 to I − 1 group was reduced 37%, which would be considered acceptable.
FIG. 3.
Cefotaxime MICs versus zone diameters with 407 S. pneumoniae isolates. Horizontal and vertical lines represent established MIC breakpoints (8) and best-fit zone diameter breakpoints, respectively.
Ceftriaxone.
The problem seen with cefotaxime is also apparent with ceftriaxone (3) (Fig. 4).The major and very major discrepancy rates are similar to those of cefotaxime, but the minor discrepancy rate in the I + 1 to I − 1 group is even higher (71%).
Conclusions.
The newer NCCLS guidelines for calculating discrepancy rates led us to the following conclusions: (i) the established zone diameter breakpoints for erythromycin are appropriate; (ii) acceptable zone diameter breakpoints for telithromycin could readily be set, if we assume that tentative MIC breakpoints are proven to be acceptable; and (iii) the minor discrepancy rates for cefotaxime and ceftriaxone disk tests are too excessive to consider testing these 30-μg disks against pneumococci. It is conceivable that a smaller disk content (e.g., 1 μg) might produce more reliable results.
We believe the latest NCCLS guidelines for setting zone diameter breakpoints are a step in the right direction. The acceptable discrepancy rate limits may need to be fine-tuned in the future as more experience is gained with this method. Judgment will clearly be called for when certain discrepancy rates exceed the current limits. For example, if there is one very major discrepancy in the ≥I + 2 group and there are only 10 isolates in this group (10% discrepancy rate), it would be reasonable to waive the 2% limit if all else fits well.
Acknowledgments
This work was supported in part by grants from Aventis Pharmaceuticals and Roche Laboratories.
REFERENCES
- 1.Barry, A. L., S. D. Brown, and W. J. Novick. 1995. Criteria for testing the susceptibility of Streptococcus pneumoniae to cefotaxime and its desacetyl metabolite using 1 μg and 30 μg disks. Eur. J. Clin. Microbiol. Infect. Dis. 14:724-726. [DOI] [PubMed] [Google Scholar]
- 2.Brunden, M. N., G. E. Zurenko, and B. Kapik. 1992. Modification of the error-bounded classification scheme for use with two MIC breakpoints. Diagn. Microbiol. Infect. Dis. 15:135-140. [DOI] [PubMed] [Google Scholar]
- 3.Jorgensen, J. H., J. M. Swenson, F. C. Tenover, M. J. Ferraro, J. A. Hindler, and P. R. Murray. 1994. Development of interpretive criteria and quality control limits for broth microdilution and disk diffusion antimicrobial susceptibility testing of Streptococcus pneumoniae. J. Clin. Microbiol. 32:2448-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Metzler, D. M., and R. M. DeHaan. 1974. Susceptibility tests of anaerobic bacteria: statistical and clinical considerations. J. Infect. Dis. 130:588-594. [DOI] [PubMed] [Google Scholar]
- 5.National Committee for Clinical Laboratory Standards. 1994. Development of in vitro susceptibility testing criteria and quality control parameters. Approved standard M23-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 6.National Committee for Clinical Laboratory Standards. 2001. Development of in vitro susceptibility testing criteria and quality control parameters. Approved standard M23-A2. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 7.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 8.National Committee for Clinical Laboratory Standards. 2000. Performance standards for antimicrobial disk susceptibility tests. Approved standard M2-A7. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 9.National Committee for Clinical Laboratory Standards. 2000. Performance standards for antimicrobial susceptibility testing. Tenth informational supplement M100-S10. National Committee for Clinical Laboratory Standards, Wayne, Pa.