Abstract
Caspofungin is an antifungal agent of the novel echinocandin class. We investigated its efficacy, safety, and tolerability as therapy for oropharyngeal and/or esophageal candidiasis in a phase II dose-ranging study. Patients were randomized in a double-blind manner to receive either caspofungin acetate (35, 50, or 70 mg) or amphotericin B (0.5 mg/kg of body weight) intravenously once daily for 7 to 14 days. A favorable response required both complete resolution of symptoms and quantifiable improvement of mucosal lesions 3 to 4 days after discontinuation of study drug. Efficacy was assessed using a modified intent-to-treat analysis. No hypothesis testing of efficacy was planned or performed. Of 140 enrolled patients, 63% had esophageal involvement and 98% were infected with the human immunodeficiency virus (HIV) (median CD4 count, 30/mm3). A modestly higher proportion of patients in each of the caspofungin groups (74 to 91%) achieved favorable responses compared to amphotericin B recipients (63%), but there was considerable overlap in the 95% confidence intervals surrounding these point estimates. Similar trends were found in the subgroups with esophageal involvement, a history of fluconazole failure, and CD4 counts of ≤50/mm3. A smaller proportion of patients receiving any dose of caspofungin experienced drug-related adverse events compared to patients given standard doses of conventional amphotericin B (P < 0.01). Caspofungin provided a generally well-tolerated parenteral therapeutic option for HIV-infected patients with oropharyngeal and/or esophageal candidiasis in this study.
Mucosal candidiasis, although not life-threatening, causes significant morbidity in patients infected with the human immunodeficiency virus (HIV) (16). The development of drug resistance in the causative strain or the selection of intrinsically more resistant species may complicate therapy of recurrent candidal infections (19, 20, 28, 34, 36, 40, 43). This study describes the use of a new echinocandin, caspofungin (Cancidas; formerly MK-0991), as treatment for oropharyngeal and esophageal candidiasis in an immunocompromised patient population.
Oral fluconazole is widely regarded as the treatment of choice for mucosal candidiasis under most circumstances (36). Amphotericin B deoxycholate has been the standard recourse for patients infected with Candida sp. unresponsive to azole therapy (5, 10, 24, 36). The use of conventional amphotericin B preparations is complicated by its significant toxicity. Newer lipid formulations of amphotericin have reduced, but not eliminated, some of these adverse effects (15).
Caspofungin possesses activity in vitro (6, 17, 23, 30, 31, 42) and in vivo (1, 2, 11, 13) against a variety of fungal pathogens, including Candida and Aspergillus species. Echinocandins noncompetitively inhibit 1,3-β-d-glucan synthesis, interfering with the normal formation of the fungal cell wall (3). Since glucans are not present in mammalian cells, it is hoped that echinocandins will have a relatively high therapeutic index. Cross-resistance with conventional antifungal compounds is not anticipated because of their unique mechanism of action.
(Preliminary results from this study were presented in November 1998 at the Infectious Disease Society of America meeting in Denver, Colo.)
MATERIALS AND METHODS
Study population and design.
We conducted a randomized, double-blind, four-arm study of three different doses of caspofungin and standard dose amphotericin B therapy for patients with symptomatic oropharyngeal and/or esophageal candidiasis. The protocol was approved by the institutional review boards of the 18 participating centers. Informed consent was obtained from all enrolled patients. Patients between 18 and 65 years of age were eligible for enrollment if they had a diagnosis of oropharyngeal and/or esophageal candidiasis documented by visualization of Candida pseudohyphae in appropriate specimens. Women of child-bearing potential were eligible only if they had a negative serum pregnancy test, were not breast-feeding, and agreed to use adequate contraception throughout the study period. Exclusion criteria included a history of allergy or serious adverse reaction to glucan synthesis inhibitors or amphotericin B, previous failure of amphotericin B therapy for oropharyngeal and/or esophageal candidiasis, ongoing treatment with rifampin or ritonavir, and any underlying condition deemed likely to confound interpretation of the results or pose undue risk to the patient. Abnormal laboratory values that disqualified patients from participating in this study were hematocrit of ≤27%; absolute neutrophil count of <1,000/μl; platelet count of ≤75,000/μl; creatinine clearance of <50 ml/min; prothrombin time greater than the upper limit of normal (ULN); and/or total levels of bilirubin in serum of three or more times the ULN, levels of aspartate or alanine aminotransferase of five or more times the ULN, or levels of alkaline phosphatase in serum of three or more times the ULN. Patients were randomly allocated to receive caspofungin acetate (35, 50, or 70 mg or a matching placebo), followed by amphotericin B (0.5 mg/kg of body weight or a matching placebo) intravenously once daily and stratified according to presentation with either oropharyngeal infection alone or esophageal disease with or without oropharyngeal involvement. Patients were further stratified as to whether their infections had previously been refractory (substratum I) or responsive (substratum II) to fluconazole therapy. Patients never previously exposed to fluconazole were presumed to have fluconazole-responsive infections. Each patient received 1 active drug and a placebo. Caspofungin or matching placebo was infused over 1 h, followed by infusion of amphotericin B or matching placebo given over 2 h. Premedication, typically consisting of acetaminophen and/or diphenhydramine, was administered to patients who had experienced infusion-associated chills, fever, or tachypnea when receiving an initial test dose of study drug or during subsequent doses. Patients with isolated oropharyngeal candidiasis were treated for a minimum of 7 days, while patients with esophageal involvement were treated for a minimum of 10 days. The maximum duration of treatment was 14 days.
Evaluation of drug efficacy.
The primary measure of efficacy was prespecified as the combined response of referable symptoms and visible lesions assessed 3 to 4 days after discontinuation of study drug. Esophageal lesions were graded endoscopically as follows: grade 0, normal mucosa; grade 1/2, scattered individual plaques <2 mm in size; grade 1, scattered plaques >2 mm in size covering <50% of the mucosa; grade 2, plaques >2 mm in size covering >50% of the mucosa; grade 3, confluent plaques circumferentially covering ≥50% of the mucosa; and grade 4, circumferential plaques with narrowing despite insufflation (4). Oropharyngeal lesions were assessed by routine physical examination as follows: grade 0, no lesions present; grade 1, scattered, nonconfluent lesions, with the majority <2 mm in size; grade 2, multiple lesions >2 mm in size; and grade 3, extensive, nearly confluent lesions. Clinical responses were rated as favorable only if the patient was asymptomatic at the primary endpoint. The response of esophageal lesions was assessed by repeat endoscopy. Favorable responses were defined for patients with baseline grades of ≥2 as an endpoint grade of ≤1/2; if the baseline grade was 1/2 or 1, an endpoint grade of 0 was required for a favorable response. The response of oropharyngeal lesions was regarded as favorable only if the endpoint grade was 0. The combined response represented the primary endpoint and was recorded as favorable if and only if both components were favorable. When data were missing, the combined response was considered unfavorable in the modified intention-to-treat (MITT) analysis and excluded from the evaluable-patients analysis (see below). The esophageal outcome was the determinant of response in patients with both esophageal and oropharyngeal disease. For those patients who had a favorable response at the primary endpoint, relapse was defined as the recurrence of symptoms or signs of Candida infection during the month after discontinuation of therapy. Microbiological eradication was assessed as a secondary measure of efficacy based upon the results of cultures 3 to 4 days after discontinuation of therapy. When cultures were not performed but the patient had no evidence of residual infection, eradication was presumed.
Assessment of drug safety and tolerability.
Patients were monitored for clinical adverse experiences on a daily basis during the study and at follow-up assessments after discontinuation of therapy. Periodic monitoring of laboratory tests was also performed throughout the study period. Adverse events were rated by the investigator as to their severity and the likelihood of their relationship to the study medication. The local tolerability of infusions was assessed daily as follows: well tolerated (no evidence of irritation); moderately well tolerated (mild to moderate erythema or discomfort); and poorly tolerated (prominent erythema, pain, swelling, or thrombophlebitis).
Statistical analysis.
This trial was designed as a dose-ranging estimation study and was not explicitly powered to test whether treatment groups differed significantly with respect to efficacy. Accordingly, hypothesis testing of the relative efficacy of the various treatment regimens was not performed. For each measure of efficacy, data were expressed as an observed proportion with its 95% confidence interval. The primary analysis of efficacy was based on combined clinical and endoscopic response rate in the MITT population 3 to 4 days after discontinuation of study drug. The MITT population consisted of randomized patients who received at least one dose of study drug. The evaluable-patient population consisted of patients who received at least 5 days of study medication, did not take concomitant antifungal drugs from baseline through the 3- to 4-day-posttherapy visit, completed their follow-up evaluation, and had no serious protocol violations. For the safety analysis, pairwise comparisons of the frequency of clinical and laboratory adverse events between any of the caspofungin groups and the amphotericin B group were analyzed using Fisher's exact test without correction for multiplicity.
RESULTS
Patient characteristics on entry.
The majority of the 140 enrolled patients were male (79%) and of Hispanic or mestizo origin (69%). Ages ranged from 18 to 65 years. The demographic characteristics for the four treatment groups were similar. Disease characteristics were also balanced across treatment groups with respect to stratum, substratum, and baseline endoscopic or oropharyngeal grades (Table 1). Only three patients in the entire study were HIV seronegative. Candida species were recovered from 126 patients (90%). The majority of infections (100 of 126 patients; 79%) were caused by Candida albicans alone. Candida guilliermondii and Candida tropicalis were solitary pathogens in two patients. The remaining 24 cases were mixed infections, usually involving C. albicans and another species. Two patients, both assigned to the caspofungin 70-mg group, were excluded from the MITT analysis because of either the absence of symptoms or microbiological documentation.
TABLE 1.
Baseline patient characteristics by treatment group
| Characteristic | Value for patients exhibiting characteristica
|
Total patients (n = 140) | |||
|---|---|---|---|---|---|
| Caspofungin acetate, 35 mg (n = 34) | Caspofungin acetate, 50 mg (n = 34) | Caspofungin acetate, 70 mg (n = 37) | Amphotericin B, 0.5 mg/kg (n = 35) | ||
| Stratumb | |||||
| Esophageal (± oropharyngeal) | 21 (61.8) | 20 (58.8) | 24 (64.9) | 23 (65.7) | 88 (62.9) |
| Oropharyngeal (exclusively) | 13 (38.2) | 14 (41.2) | 13 (35.1) | 12 (34.3) | 52 (37.1) |
| Substratumc | |||||
| Unresponsive to fluconazole | 6 (17.6) | 3 (8.8) | 7 (18.9) | 5 (14.3) | 21 (15.0) |
| Responsive to fluconazoled | 28 (82.4) | 31 (91.2) | 30 (81.1) | 30 (85.7) | 119 (85.0) |
| Stratum-substratume | |||||
| 1 | 2 (5.9) | 1 (2.9) | 2 (5.4) | 1 (2.9) | 6 (4.3) |
| 2 | 11 (32.4) | 13 (38.2) | 11 (29.7) | 11 (31.4) | 46 (32.9) |
| 3 | 4 (11.8) | 2 (5.9) | 5 (13.5) | 4 (11.4) | 15 (10.7) |
| 4 | 17 (50.0) | 18 (52.9) | 19 (51.4) | 19 (54.3) | 73 (52.1) |
| HIV infection | |||||
| Positive | 32 (94.1) | 33 (97.1) | 37 (100.0) | 35 (100.0) | 137 (97.9) |
| Negative | 2 (5.9) | 1 (2.9) | 0 (0.0) | 0 (0.0) | 3 (2.1) |
| Baseline esophageal grade | |||||
| 1/2 | 1 (2.9) | 2 (5.9) | 2 (5.4) | 3 (8.6) | 8 (5.7) |
| 1 | 4 (11.8) | 0 (0.0) | 2 (5.4) | 1 (2.9) | 7 (5.0) |
| 2 | 3 (8.8) | 5 (14.7) | 7 (18.9) | 3 (8.6) | 18 (12.9) |
| 3 | 10 (29.4) | 11 (32.4) | 9 (24.3) | 12 (34.3) | 42 (30.0) |
| 4 | 2 (5.9) | 2 (5.9) | 4 (10.8) | 4 (11.4) | 12 (8.6) |
| Baseline oropharyngeal grade | |||||
| 1 | 6 (17.6) | 6 (17.6) | 2 (5.4) | 4 (11.4) | 18 (12.9) |
| 2 | 3 (8.8) | 8 (23.5) | 7 (18.9) | 4 (11.4) | 22 (15.7) |
| 3 | 4 (11.8) | 0 (0.0) | 4 (10.8) | 4 (11.4) | 12 (8.6) |
| CD4 countf | |||||
| No. of patients | 31 | 32 | 36 | 34 | 133 |
| Mean | 109.2 | 72.6 | 51.9 | 59.4 | 72.2 |
| SD | 198.1 | 99.8 | 102.4 | 65.9 | 124.9 |
| Median | 48.0 | 34.0 | 21.5 | 29.0 | 30.0 |
| Range | 2-879 | 0-405 | 0-561 | 0-260 | 0-879 |
Results are presented as number (percent) unless otherwise indicated.
Primary site of infection.
Prior response to fluconazole.
Includes patients not previously exposed to fluconazole or other azole antifungal agents.
Stratum-substratum categories: 1, oropharyngeal candidiasis alone and unresponsive to fluconazole; 2, oropharyngeal candidiasis alone and responsive to or not previously exposed to fluconazole; 3, esophageal candidiasis (with or without oropharyngeal candidiasis) and unresponsive to fluconazole; 4, esophageal candidiasis (with or without oropharyngeal candidiasis) and responsive or not previously exposed to fluconazole.
CD4 count tabulations include only those patients with HIV infection. Mean, standard deviation, median, and range are given as number of cells per cubic millimeter.
Efficacy.
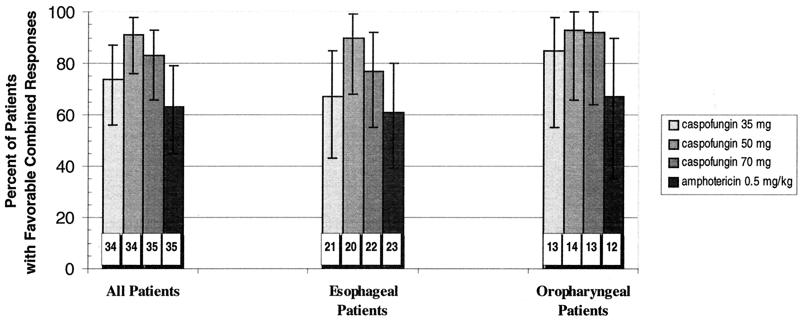
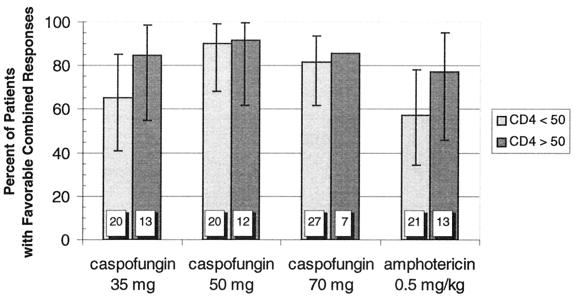
A modestly higher proportion of patients treated with caspofungin than amphotericin B achieved a favorable combined response in the MITT analysis (Fig. 1).Similar trends were observed in the subgroups of patients with esophageal and oropharyngeal involvement. In patients with ≤50 CD4 cells/mm3, favorable responses occurred more often with the higher two doses of caspofungin (18 of 20 patients [90%] and 22 of 27 patients [82%] for 50 and 70 mg, respectively) than the 35-mg dose (13 of 20 patients [65%]) or amphotericin (12 of 21 patients [57%]) (Fig. 2).In each case, the 95% confidence intervals around the point estimates of efficacy for the various treatment arms overlapped; therefore, our data do not suggest therapeutic superiority for any of the drug regimens tested.
FIG. 1.
Combined favorable response rates in the caspofungin and amphotericin groups. Patients presenting with either esophageal or oropharyngeal candidiasis were treated with caspofungin (35, 50, and 70 mg) or amphotericin (0.5 mg/kg) daily for 7 to 14 days. Patients were evaluated 3 to 4 days after discontinuation of therapy. Numbers within the columns represent the total number of patients in each group included in the MITT analysis. Error bars represent the 95% confidence intervals.
FIG. 2.
Combined favorable response rates to caspofungin and amphotericin therapy by baseline CD4 lymphocyte count. CD4 cell counts were measured at screening. Numbers within the columns represent the total number of patients in each group. Error bars represent the 95% confidence intervals. The confidence interval was not calculated for the caspofungin 70-mg dose group having >50 CD4 cells/mm3 because the sample size was only seven patients.
In total, more patients with exclusively oropharyngeal disease (85%) than with esophageal involvement (73%) had favorable combined responses. The time to symptom resolution did not differ among treatment groups. Symptoms abated more quickly on the average in patients with exclusively oropharyngeal disease (median time, 2 to 3 days) than in patients with esophageal involvement (median time, 3 to 4 days). Across all treatment regimens, patients with a history of fluconazole-refractory infection responded less often (38% for substratum I) than patients with presumptively fluconazole-susceptible infection (85% for substratum II), but there were relatively few patients (n = 21) in substratum I.
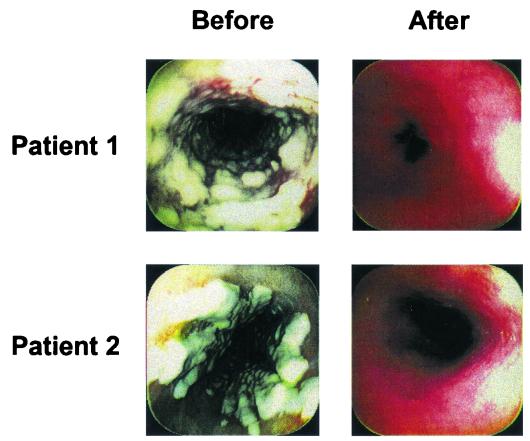
Patients were assessed by endoscopic or oropharyngeal examination for reduction in the size and extent of mucosal lesions, as illustrated for two patients given caspofungin in Fig. 3.In the MITT analysis, the proportion of esophageal patients with a favorable endoscopic response at the 3- to 4-day follow-up evaluation was numerically greater in the caspofungin groups (67, 90, and 77% for the 35-, 50-, and 70-mg dose levels, respectively) than in the amphotericin B group (61%). Comparable differences between caspofungin and amphotericin B were also observed in patients whose infections were confined to the oropharynx (84, 93, and 92% versus 67%). Microbiological eradication was seen in a larger proportion of patients treated with caspofungin (at all doses) compared with amphotericin B. Caspofungin cleared C. albicans in more than 75% of patients in each dosing group, compared to 55% for the amphotericin B patients. However, this study was not large enough to formally compare the relative efficacy in different subgroups, and no conclusions can be drawn from our data in this regard. Overall, there was no significant difference in the clearance of C. albicans (74%) versus non-C. albicans (81%) species. Relapse rates in the month following discontinuation of study drug were as high as 37% and were similar among treatment groups.
Safety and tolerability.
The incidence of drug-related clinical adverse experiences was significantly less (P < 0.01) in each of the caspofungin arms than in the amphotericin B group (Table 2). Significantly fewer caspofungin recipients developed drug-related fever, chills, nausea, or vomiting. Patients receiving amphotericin required premedication for infusion-related side effects more frequently than patients receiving caspofungin at any dose (69% versus <1%). The incidence of local reactions ranged from 6 to 14% across treatment arms, but all patients except one amphotericin recipient were judged to have tolerated the study drug at least moderately well. No patient in the caspofungin groups had a serious drug-related clinical adverse experience, in contrast to three patients (9%) receiving amphotericin B. Two patients in the caspofungin 35-mg dose group and one patient in the amphotericin B group discontinued treatment because of drug-related clinical adverse experiences.
TABLE 2.
Summary of adverse experiences
| Treatment group | No. (%) of patients experiencing adverse event
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Clinical
|
Laboratory
|
|||||||
| Drug relateda | Serious and drug relateda | Therapy stopped due to a serious drug-relateda clinical adverse event | Died from any cause | Drug relateda | Serious and drug relateda | Therapy stopped due to a serious drug-relateda laboratory adverse event | ||
| Caspofungin acetate, 35 mg (n = 34) | 17 (50)b | 0 (0) | 0 (0) | 3 (9) | 16 (48) | 0 (0) | 0 (0) | |
| Caspofungin acetate, 50 mg (n = 34) | 12 (35)b | 0 (0) | 0 (0) | 1 (3) | 15 (44)c | 0 (0) | 0 (0) | |
| Caspofungin acetate, 70 mg (n = 37) | 17 (46)b | 0 (0) | 0 (0) | 3 (8) | 13 (35)b | 0 (0) | 0 (0) | |
| Amphotericin B, 0.5 mg/kg (n = 35) | 34 (97) | 3 (9) | 1 (3) | 5 (14) | 25 (71) | 1 (3) | 0 (0) | |
| All patients (n = 140)d | 80 (57) | 3 (2) | 1 (1) | 12 (9) | 69 (50) | 1 (1) | 0 (0) | |
Determined by the investigator to be possibly, probably, or definitely drug related.
P ≤ 0.01 versus amphotericin B.
P ≤ 0.05 versus amphotericin B.
Only 33 of the 34 patients in the caspofungin, 35 mg, group had follow-up laboratory results and were included in the tabulation of laboratory adverse events.
Drug-related laboratory abnormalities were also more common in patients taking amphotericin B than in patients receiving caspofungin (Table 3), the differences reaching statistical significance for comparisons between the caspofungin 50-mg (P < 0.05) and 70-mg (P < 00.01) dose groups versus the amphotericin recipients. The most common drug-related laboratory abnormalities in patients receiving caspofungin were elevations in serum levels of alanine aminotransferase, aspartate aminotransferase, and alkaline phosphatase, which were typically less than five times the ULN and resolved despite continued treatment. None of the patients receiving caspofungin and nine patients (26%) given amphotericin B developed drug-related increases in serum creatinine levels (P < 0.01 for pairwise comparisons between each of the caspofungin groups and the amphotericin B group). Drug-related hypokalemia was reported in 1 patient (3%) given caspofungin, 35 mg; 1 patient (3%) given caspofungin, 50 mg; 4 patients (11%) given caspofungin, 70 mg; and 13 patients (37%) given amphotericin B (P < 0.01 for comparison of caspofungin [35- and 50-mg doses] and amphotericin B and P < 0.05 for comparison of caspofungin [70-mg dose] and amphotericin B). The only serious drug-related laboratory abnormality occurred in the amphotericin B group. No patient stopped therapy because of drug-related laboratory adverse experiences.
TABLE 3.
| Symptom | Caspofungin acetatec
|
Amphotericin B, 0.5 mg/kg (n = 35) | |||
|---|---|---|---|---|---|
| 35 mg (n = 34) | 50 mg (n = 34) | 70 mg (n = 37) | |||
| n (%) | n (%) | n (%) | n (%) | ||
| Chills | 1 (2.9)** | 1 (2.9)** | 1 (2.7)** | 28 (80.0) | |
| Fever | 7 (20.6)** | 4 (11.8)** | 6 (16.2)** | 25 (71.4) | |
| Phlebitis | 3 (8.8) | 1 (2.9) | 2 (5.4) | 5 (14.3) | |
| Diarrhea | 1 (2.9) | 0 (0.0) | 1 (2.7) | 5 (14.3) | |
| Nausea | 3 (8.8)* | 1 (2.9)** | 1 (2.7)** | 11 (31.4) | |
| Vomiting | 0 (0.0)** | 0 (0.0)** | 1 (2.7)* | 8 (22.9) | |
| Rash | 3 (8.8) | 0 (0.0) | 0 (0.0) | 1 (2.9) | |
| Headache | 4 (11.8) | 2 (5.9) | 2 (5.4)* | 8 (22.9) | |
Specific adverse events occurring in ≥7.5% of patients in any treatment group.
Determined by the investigator to be possibly, probably, or definitely drug related.
∗, P ≤ 0.05 versus amphotericin B; ∗∗, P ≤ 0.01 versus amphotericin B.
DISCUSSION
Oropharyngeal and esophageal candidiases still account for significant suffering and disability among patients with advanced HIV infection (16). These infections are characterized by their propensity to recur (20). Over time, especially with repeated courses of fluconazole therapy in patients with CD4 lymphocyte counts below 50/mm3 (19, 28, 34, 43), favorable treatment responses become less likely (14, 18, 22, 24, 29, 33, 35, 38). Approaches to recalcitrant infections have included escalating the dose of fluconazole (36, 37, 38), using intravenous instead of oral administration (36), switching to another member of the azole class (7, 32, 39), adding topical antifungal agents (7, 9, 21, 26, 27, 39, 41, 42), changing to conventional or lipid preparations of amphotericin B (36), or trying investigational drugs (17, 25, 31, 42; S. P. Bachman, S. Perea, W. R. Kirkpatrick, T. F. Patterson, and J. L. Lopez-Ribot, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., p. 352, 2000). In this context, the echinocandins provide a new class of antifungal agents that uniquely inhibit the synthesis of cell wall glucans critical to the integrity of many yeasts and molds (3). Cross-resistance with conventional antifungal agents is unlikely, because echinocandins work through an unrelated mechanism (12).
In the present study, concurrent resolution of symptoms and improvement of mucosal lesions occurred more often in patients receiving any of the three doses of caspofungin than in the amphotericin B group. However, this dose-ranging trial was not designed to show therapeutic noninferiority of either drug. In patients with CD4 counts below 50 cells/mm3, the higher doses (50 and 70 mg) of caspofungin produced a numerically greater proportion of favorable responses than either amphotericin B or the lower dose (35 mg) of caspofungin. A dose of 50 mg/day appeared as effective as 70 mg/day. There was no apparent dose-related toxicity over this dosing range of caspofungin. On this basis, a caspofungin dose of 50 mg/day was chosen for future studies.
There was a lower response rate for both caspofungin and amphotericin in the small number of patients previously failing fluconazole therapy than in patients without such a history. Since both drugs have mechanisms of action unrelated to that of the azoles, fluconazole resistance per se was probably not the main determinant of outcome in this substratum. Failure to respond to fluconazole in the past more likely represented a nonspecific marker for increased immunosuppression and other host factors (8, 12).
Caspofungin therapy was generally well tolerated. The incidence of drug-related clinical and laboratory adverse events was significantly less in all of the caspofungin groups relative to the amphotericin B recipients. The types of adverse events most commonly associated with the administration of caspofungin were fever, headache, nausea, phlebitis, rash, and elevations in hepatic enzyme levels, which rarely led to discontinuation of therapy. Premedication was required more often before amphotericin B than caspofungin infusions. Unlike amphotericin B therapy, treatment with caspofungin was only rarely associated with drug-induced deterioration of renal function. Short-term trials cannot fully address the potential impact of the differences in nephrotoxicity between these two drugs.
In HIV-infected patients with esophageal and/or oropharyngeal candidiasis, caspofungin appeared to possess an efficacy at least comparable to that of a standard dose of amphotericin B. Our results further suggest that caspofungin may provide a better-tolerated alternative option to conventional amphotericin for patients who require parenteral therapy, such as those with azole-refractory Candida infections.
FIG. 3.
Resolution of esophageal plaques caused by infection with C. albicans in two patients treated with caspofungin. Favorable endoscopic responses are illustrated for two representative patients with high-grade esophagitis 3 to 4 days after the conclusion of caspofungin therapy.
Acknowledgments
We recognize and appreciate the contributions of Jorge Andrade (Guadalajara, Mexico), J. Richard Graybill (San Antonio, Tex.), Jose Vasquez (Detroit, Mich.), David Uip (Sao Paulo, Brazil), David Barker (Chicago, Ill.), Gary Cox (Durham, N.C.), John Rex (Houston, Tex.), Meera Kashkari-Kelley (Chapel Hill, N.C.), Princy Kumar (Washington, D.C.), John Bennett (Bethesda, Md.), Annette Reboli (Camden, N.J.), Marla Gold (Philadelphia, Pa.), Cecilia Shikuma (Honolulu, Hawaii), Bernard McNamara (Los Angeles, Calif.), and Roy Steigbigel (Stony Brook, N.Y.) in enrolling patients into this study. We also thank Arlene Taylor, Robert Lupinacci, Mary Motyl, and Jeffery Chodakewitz for their suggestions and Joann DiLullo for her expert assistance in preparing the manuscript.
REFERENCES
- 1.Abruzzo, G. K., C. J. Gill, A. M. Flattery, L. Kong, C. Leighton, J. G. Smith, V. B. Pikounis, K. Bartizal, and H. Rosen. 2000. Efficacy of the echinocandin caspofungin against disseminated aspergillosis and candidiasis in cyclophosphamine-induced immunosuppressed mice. Antimicrob. Agents Chemother. 44:2310-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abruzzo, G. K., A. M. Flattery, C. J. Gill, L. Kong, J. G. Smith, V. B. Pikounis, et al. 1997. Evaluation of the echinocandin antifungal MK-0991 (L-743,872): efficacies in mouse models of disseminated aspergillosis, candidiasis, and cryptococcosis. Antimicrob. Agents Chemother. 41:2333-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andriole, V. T. 1999. Current and future antifungal therapy: new targets for antifungal agents. J. Antimicrob. Chemother. 44:151-162. [DOI] [PubMed] [Google Scholar]
- 4.Baehr, P. H., and G. B. McDonald. 1994. Esophageal infections: risk factors, presentation, diagnosis, and treatment. Gastroenterology 106:509-532. [DOI] [PubMed] [Google Scholar]
- 5.Barchiesi, F., A. L. Colombo, D. A. McGough, A. W. Fothergill, and M. G. Rinaldi. 1994. In vitro activity of itraconazole against fluconazole-susceptible and -resistant Candida albicans isolates from oral cavities of patients infected with human immunodeficiency virus. Antimicrob. Agents Chemother. 38:1530-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barchiesi, F., A. M. Schimizzi, A. W. Fothergill, G. Scalise, and M. G. Rinaldi. 1999. In vitro activity of the new echinocandin antifungal, MK-0991, against common and uncommon clinical isolates of Candida species. Eur. J. Clin. Microbiol. Infect. Dis. 18:302-304. [DOI] [PubMed] [Google Scholar]
- 7.Eichel, M., G. Just-Nubling, E. B. Helm, and W. Stille. 1996. Itraconazole suspension in the treatment of HIV-infected patients with fluconazole-resistant oropharyngeal candidiasis and esophagitis. Mycoses. 39(Suppl. 1):102-106. [DOI] [PubMed] [Google Scholar]
- 8.Fichtenbaum, C. J., and W. G. Powderly. 1998. Refractory mucosal candidiasis in patients with human immunodeficiency virus infection. Clin. Infect. Dis. 26:556-565. [DOI] [PubMed] [Google Scholar]
- 9.Fichtenbaum, C. J., R. Zackin, N. Rajicic, W. G. Powderly, L. J. Wheat, and B. S. Zingman. 2000. Amphotericin B oral suspension for fluconazole-refractory oral candidiasis in persons with HIV infection. AIDS 14:845-852. [DOI] [PubMed] [Google Scholar]
- 10.Goldman, M., G. A. Cloud, M. Smedema, A. LeMonte, P. Conolly, D. S. McKinsey, C. A. Kauffman, B. Moskovitz, and L. J. Wheat. 2000. Does long-term itraconazole prophylaxis result in in vitro azole resistance in mucosal Candida albicans isolates from persons with advanced human immunodeficiency virus infection? Antimicrob. Agents Chemother. 44:1585-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graybill, J. R., R. Bocanegra, M. Luther, A. Fothergill, and M. J. Rinaldi. 1997. Treatment of murine Candida krusei or Candida glabrata infection with L-743,872. Antimicrob. Agents Chemother. 41:1937-1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graybill, J. R., E. Montalbo, W. R. Kirkpatrick, M. F. Luther, S. G. Revankar, and T. F. Patterson. 1998. Fluconazole versus Candida albicans: a complex relationship. Antimicrob. Agents Chemother. 42:2938-2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graybill, J. R., J. K. Najvar, M. F. Luther, and A. W. Fothergill. 1997. Treatment of murine disseminated candidiasis with L-743,872. Antimicrob. Agents Chemother. 41:1775-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heald, A. E., G. M. Cox, W. A. Schell, J. A. Bartlett, and J. R. Perfect. 1996. Oropharyngeal yeast flora and fluconazole resistance in HIV-infected patients receiving long-term continuous versus intermittent fluconazole therapy. AIDS 10:263-268. [DOI] [PubMed] [Google Scholar]
- 15.Hiemenz, J. W., and T. J. Walsh. 1996. Lipid formulations of amphotericin B: recent progress and future directions. Clin. Infect. Dis. 22(Suppl. 2):S133-S144. [DOI] [PubMed]
- 16.Kaplan, J. E., D. Hanson, M. S. Dworkin, T. Frederick, J. Bertolli, M. L. Lindegren, S. Holmberg, and J. L. Jones. 2000. Epidemiology of human immunodeficiency virus-associated opportunistic infections in the United States in the era of highly active antiretroviral therapy. Clin. Infect. Dis. 30(Suppl. 1):55-114. [DOI] [PubMed] [Google Scholar]
- 17.Krishnarao, T. V., and J. N. Galgiani. 1997. Comparison of the in vitro activities of the echinocandin LY303366, the pneumocandin MK-0991, and fluconazole against Candida species and Cryptococcus neoformans. Antimicrob. Agents Chemother. 41:1957-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lacassin, F., F. Damond, C. Chochillon, P. Longuet, J. Lebras, J. L. Vilde, and C. Leport. 1996. Response to fluconazole by 23 patients with human immunodeficiency virus infection and oral candidiasis: pharmacological and mycological factors. Antimicrob. Agents Chemother. 40:1961-1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laguna, F., J. L. Rodriguez-Tudela, J. V. Martinez-Suarez, R. Polo, E. Valencia, T. M. Diaz-Guerra, F. Dronda, and F. Pulido. 1997. Patterns of fluconazole susceptibility in isolates from human immunodeficiency virus-infected patients with oropharyngeal candidiasis due to Candida albicans. Clin. Infect. Dis. 24:124-130. [DOI] [PubMed] [Google Scholar]
- 20.Laine, L. 1994. The natural history of esophageal candidiasis after successful treatment in patients with AIDS. Gastroenterology 107:744-746. [DOI] [PubMed] [Google Scholar]
- 21.Lalor, E., and L. Rabeneck. 1991. Esophageal candidiasis in AIDS. Successful therapy with clotrimazole vaginal tablets taken by mouth. Dig. Dis. Sci. 36:279-281. [DOI] [PubMed] [Google Scholar]
- 22.Landman, D., G. Saurina, and J. M. Quale. 1998. Failure of all antifungal therapy for infection due to Candida albicans: a new AIDS-related problem? Clin. Infect. Dis. 26:183-184. [DOI] [PubMed] [Google Scholar]
- 23.Marco, F., M. A. Pfaller, S. A. Messer, and R. N. Jones. 1998. Activity of MK-0991 (L-743,872), a new echinocandin, compared with those of LY303366 and four other antifungal agents tested against blood stream isolates of Candida spp. Diagn. Microbiol. Infect. Dis. 32:33-37. [DOI] [PubMed] [Google Scholar]
- 24.Martinez-Suarez, J. V., and J. L. Rodriguez-Tudela. 1995. Patterns of in vitro activity of itraconazole and imidazole antifungal agents against Candida albicans with decreased susceptibility to fluconazole from Spain. Antimicrob. Agents Chemother. 39:1512-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinez-Suarez, J. V., and J. L. Rodriguez-Tudela. 1996. In vitro activities of semisynthetic pneumocandin L-733,560 against fluconazole-resistant and -susceptible Candida albicans isolates. Antimicrob. Agents Chemother. 40:1277-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martins, M. D., and J. H. Rex. 1997. Fluconazole suspension for oropharyngeal candidiasis unresponsive to tablets. Ann. Intern. Med. 126:332-333. [DOI] [PubMed] [Google Scholar]
- 27.Mascarenas, C. A., T. C. Hardin, G. J. Pennick, M. G. Rinaldi, and J. R. Graybill. 1998. Treatment of thrush with itraconazole solution: evidence for topical effect. Clin. Infect. Dis. 26:1242-1243. [DOI] [PubMed] [Google Scholar]
- 28.Masia, C. M., R. F. Guiterrez, and D. V. Ortiz de la Tabla. 2000. Determinants for the development of oropharyngeal colonization or infection by fluconazole-resistant Candida strains in HIV-infected patients. Eur. J. Clin. Microbiol. Infect. Dis. 19:593-601. [DOI] [PubMed] [Google Scholar]
- 29.Newman, S. L., T. P. Flanigan, A. Fisher, M. G. Rinaldi, M. Stein, and K. Vigilante. 1994. Clinically significant mucosal candidiasis resistant to fluconazole treatment in patients with AIDS. Clin. Infect Dis. 19:684-686. [DOI] [PubMed] [Google Scholar]
- 30.Pfaller, M. A., R. N. Jones, G. V. Doern, A. C. Fluit, J. Verhoef, H. S. Sader, S. A. Messer, A. Houston, S. Coffman, and R. J. Hollis. 1999. International surveillance of blood stream infections due to Candida species in the European SENTRY Program: species distribution and antifungal susceptibility including the investigational triazole and echinocandin agents. Diagn. Microbiol. Infect. Dis. 35:19-25. [DOI] [PubMed] [Google Scholar]
- 31.Pfaller, M. A., S. A. Messer, S. Gee, S. Joly, C. Pujol, D. J. Sullivan, et al. 1999. In vitro susceptibilities of Candida dubliniensis isolates tested against the new triazole and echinocandin antifungal agents. J. Clin. Microbiol. 37:870-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phillips, P., J. Zemcov, W. Mahmood, J. S. Montaner, K. Craib, and A. M. Clarke. 1996. Itraconazole cyclodextrin solution for fluconazole-refractory oropharyngeal candidiasis in AIDS: correlation of clinical response with in vitro susceptibility. AIDS 10:1369-1376. [DOI] [PubMed] [Google Scholar]
- 33.Revankar, S. G., O. P. Dib, W. R. Kirkpatrick, R. K. McAtee, A. W. Fothergill, M. G. Rinaldi, S. W. Redding, and T. F. Patterson. 1998. Clinical evaluation and microbiology of oropharyngeal infection due to fluconazole-resistant Candida in human immunodeficiency virus-infected patients. Clin. Infect. Dis. 26:960-963. [DOI] [PubMed] [Google Scholar]
- 34.Revankar, S. G., W. R. Kirkpatrick, R. K. McAtee, O. P. Dib, A. W. Fothergill, S. W. Redding, M. G. Rinaldi, and T. F. Patterson. 1996. Detection and significance of fluconazole resistance in oropharyngeal candidiasis in human immunodeficiency virus-infected patients. J. Infect. Dis. 174:821-827. [DOI] [PubMed] [Google Scholar]
- 35.Revankar, S. G., W. R. Kirkpatrick, R. K. McAtee, O. P. Dib, A. W. Fothergill, S. W. Redding, M. G. Rinaldi, S. G. Hilsenbeck, and T. F. Patterson. 1998. A randomized trial of continuous or intermittent therapy with fluconazole for oropharyngeal candidiasis in HIV-infected patients: clinical outcomes and development of fluconazole resistance. Am. J. Med. 105:7-11. [DOI] [PubMed] [Google Scholar]
- 36.Rex, J. H., T. J. Walsh, J. D. Sobel, S. G. Filler, P. G. Pappas, W. E. Dismukes, and J. E. Edwards. 2000. Practice guidelines for the treatment of candidiasis. Clin. Infect. Dis. 30:662-678. [DOI] [PubMed] [Google Scholar]
- 37.Rex, J. H., M. A. Pfaller, J. N. Galgiani, M. S. Bartlett, A. Espinel-Ingroff, M. A. Ghannoum, M. Lancaster, F. C. Odds, M. G. Rinaldi, T. J. Walsh, and A. L. Barry. 1997. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole and Candida infections. Clin. Infect. Dis. 24:235-247. [DOI] [PubMed] [Google Scholar]
- 38.Ruhnke, M., A. Eigler, I. Tennagen, B. Geiseler, E. Engelmann, and M. Tratumann. 1994. Emergence of fluconazole-resistant strains of Candida albicans in patients with recurrent oropharyngeal candidiasis and human immunodeficiency virus infection. J. Clin. Microbiol. 32:2092-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saag, M. S., W. J. Fessel, C. A. Kaufmann, K. W. Merrill, D. J. Ward, B. L. Moskovitz, C. Thomas, N. Oleka, J. A. S. Guarnieri, J. Lee, L. Brenner-Gati, and M. Llausner. 1999. Treatment of fluconazole-refractory oropharyngeal candidiasis with itraconazole oral solution in HIV-positive patients. AIDS Res. Hum. Retrovir. 15:1413-1417. [DOI] [PubMed] [Google Scholar]
- 40.Sangeorzan, J. A., S. F. Bradley, X. He, L. T. Zarins, G. L. Ridenour, R. N. Tiballi, and C. A. Kauffman. 1994. Epidemiology of oral candidiasis in HIV-infected patients: colonization, infection, treatment, and emergence of fluconazole resistance. Am. J. Med. 97:339-346. [DOI] [PubMed] [Google Scholar]
- 41.Vandercam, B., D. Gibbs, M. Baltonen, H. Jager, and O. Armignacco. 1998. Fluconazole orally dispersible tablets for the treatment of patients with oropharyngeal candidiasis. J. Int. Med. Res. 26(4):209-218. [DOI] [PubMed] [Google Scholar]
- 42.Vazquez, J. A., M. Lynch, D. Boikov, and J. D. Sobel. 1997. In vitro activity of a new pneumocandin antifungal, L-743,872, against azole-susceptible and -resistant Candida species. Antimicrob. Agents Chemother. 41:1612-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walsh, T. J., C. E. Gonzalez, S. Piscitelli, J. D. Bacher, J. Peter, R. Torres, D. Shetti, V. Katsov, K. Kligys, and C. A. Lyman. 2000. Correlation between in vitro and in vivo antifungal activities in experimental fluconazole-resistant oropharyngeal and esophageal candidiasis. J. Clin. Microbiol. 38:2369-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]