Abstract
The macrolide and levofloxacin susceptibilities of 992 isolates of Streptococcus pneumoniae from clinical specimens collected in 1999 and 2000 were determined in 10 centers in Central and Eastern European countries. The prevalences of penicillin G-intermediate (MICs, 0.125 to 1 μg/ml) and penicillin-resistant (MICs, ≤2 μg/ml) Streptococcus pneumoniae isolates were 14.3 and 16.6%, respectively. The MICs at which 50% of isolates are inhibited (MIC50s) and the MIC90s of telithromycin were 0.016 and 0.06 μg/ml, respectively; those of erythromycin were 0.06 and >64 μg/ml, respectively; those of azithromycin were 0.125 and >64 μg/ml, respectively; those of clarithromycin were 0.03 and >64 μg/ml, respectively; and those of clindamycin were 0.06 and >64 μg/ml, respectively. Erythromycin resistance was found in 180 S. pneumoniae isolates (18.1%); the highest prevalence of erythromycin-resistant S. pneumoniae was observed in Hungary (35.5%). Among erythromycin-resistant S. pneumoniae isolates, strains harboring erm(B) genes (125 strains [69.4%]) were found to be predominant over strains with mef(E) genes (25 strains [13.4%]), L4 protein mutations (28 strains [15.6%]), and erm(A) genes (2 strains [1.1%]). Similar pulsed-field gel electrophoresis patterns suggested that some strains containing L4 mutations from the Slovak Republic, Bulgaria, and Latvia were clonally related. Of nine strains highly resistant to levofloxacin (MICs, >8 μg/ml) six were isolated from Zagreb, Croatia. Telithromycin at ≤0.5 μg/ml was active against 99.8% of S. pneumoniae isolates tested and may be useful for the treatment of respiratory tract infections caused by macrolide-resistant S. pneumoniae isolates.
The increasing prevalence of antibacterial resistance among Streptococcus pneumoniae strains is a serious issue for the treatment of systemic infections (1). S. pneumoniae isolates with reduced penicillin G susceptibilities are often found to be resistant to erythromycin and other 14-membered-ring macrolides and azithromycin. In a 1997 U.S. surveillance study, the macrolide resistance rates were documented to be 30.2% among all S. pneumoniae isolates tested, 34.5% among penicillin G-intermediate strains (MICs, 0.125 to 1.0 μg/ml), 66.7% among penicillin G-resistant strains (MICs, ≥ 2.0 μg/ml), and only 4.8% among penicillin G-susceptible strains (14). Among European countries, macrolide resistance in S. pneumoniae has been reported in France (45.9%), Spain (32.6%), Belgium (31.1%), Italy (24.1%), and Switzerland (15.8%) in a study conducted in 1996 and 1997 (12).
Macrolide resistance in S. pneumoniae is usually caused by the presence of the erm(B) or mef(E) resistance determinants (17, 24, 29). The Erm(B) protein encodes 23S rRNA methylase, and most pneumococcal strains which harbor the gene are resistant to 14-, 15-, and 16-membered-ring macrolides, lincosamides, and streptogramin B (MLSB phenotype). The Mef(E) protein encodes an efflux pump that leads to resistance to only 14- and 15-membered-ring macrolides. Other mechanisms of macrolide resistance among clinical S. pneumoniae isolates have recently been described and include mutations in 23S rRNA and ribosomal protein L4 and the presence of the erm(A) [subclass e rm(TR)] gene (25, 27).
Telithromycin (HMR 3647) (8, 16, 21) is a recently developed ketolide (10, 15) which has been shown to have low MICs for both erythromycin-susceptible and erythromycin-resistant strains of S. pneumoniae. To understand macrolide susceptibility in areas where high rates of drug resistance among pneumococci have been described, Central and Eastern Europe (2), we tested the activities of telithromycin, erythromycin, azithromycin, clarithromycin, and clindamycin against 992 isolates of S. pneumoniae strains sequentially isolated from centers in 10 Central and Eastern European countries during 1999 and 2000. In order to obtain an idea of fluoroquinolone susceptibilities in this context, levofloxacin was tested as the representative fluoroquinolone.
MATERIALS AND METHODS
Bacteria and antibiotics.
Strains were consecutively isolated from the various centers during 1999 and 2000 and were screened by the optochin disk method as well as the bile solubility method for S. pneumoniae identification. Although <5% of the strains were optochin resistant, all were bile sensitive. Additionally, all strains with unusual macrolide resistance mechanisms produced the autolysin gene Lyt(A) (K. Nagai, Y. Shibasaki, K. Hasegawa, T. A. Davies, M. R. Jacobs, and P. C. Appelbaum, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 8923, 2000). The following 10 centers collected S. pneumoniae isolates from clinical specimens for the present study: Sera and Vaccine Laboratory (Warsaw, Poland), Children's Hospital of Medical Academy of Latvia (Riga, Latvia), University of Ljubljana (Ljubljana, Slovenia), National Center for Epidemiology (Budapest, Hungary), Kaunas Medical University Hospital (Kaunas, Lithuania), Institute Cantacuzino, (Bucharest, Romania), Hospital of Infectious Diseases, Medical Academy (Sofia, Bulgaria), University Hospital of Infectious Diseases (Zagreb, Croatia), National Cancer Institute (Bratislava, Slovak Republic), and National Antibiotic Reference Laboratory (Prague, Czech Republic). In all cases, duplicate organisms from different specimens from the same patient were eliminated.
All organisms were isolated in the country of origin, frozen at each center except that in Warsaw (where swabs in Amies transport medium were used), and transported on dry ice to the Hershey Medical Center, where they were stored frozen in double-strength skim milk (Difco Laboratories, Detroit, Mich.) at −70°C until use. Before the cultures were tested, they were checked for purity by colony morphology and Gram staining, and the organism identities were confirmed by optochin testing. A total of 992 S. pneumoniae isolates were identified and tested in the present study. Telithromycin was obtained from Aventis, Romainville, France. The other compounds were obtained from their respective manufacturers.
Susceptibility testing.
Susceptibility testing was performed by methods used in our laboratory on Mueller-Hinton agar (BBL Microbiology Systems, Cockeysville, Md.) supplemented with 5% sheep blood (8, 10, 21). Inocula were prepared by suspending growth from overnight cultures in Mueller-Hinton broth (BBL) to a 0.5 McFarland standard. Final inocula contained 104 CFU/spot. Plates were inoculated with a Steers replicator with 3-mm inoculating pins and were incubated overnight at 35°C in air. Because the macrolide, ketolide, azalide, and lincosamide susceptibilities of S. pneumoniae are affected by incubation in 5 to 6% CO2 (8, 11), we determined the MICs by the agar dilution method by incubation in air. All strains grew well in air and did not require CO2 for adequate growth. The lowest concentration of antibiotics that resulted in no growth was read as the MIC. Standard quality control strains, including Staphylococcus aureus ATCC 29213 and S. pneumoniae ATCC 49619, were included with each run. Breakpoints were those approved by the National Committee for Clinical Laboratory Standards for S. pneumoniae (20). For telithromycin, preliminary breakpoints of 0.5 and 2.0 μg/ml were used (C. J. Soussy, F. Goldstein, A. Bryskier, H. Drugeon, J. Andrews, F. Baquero, O. Cars, D. Felmingham, B. Olsson-Liljequist, A. Rodloff, G. C. Schito, B. Wiedemann, and R. Wise, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 321, 2000).
Determination of mechanism of macrolide resistance.
Macrolide-resistant strains were initially tested by PCR for the presence of the erm(B), mef(A) (with the primers and conditions previously described by Sutcliffe and coworkers [23]), or erm(A) gene. The erm(A) gene-specific primers used for PCR were erm(TR) for (5"-ACAGAAAAACCCGAAAAATACG-3") and erm(TR)rev (5"-TTGGATAATTTATCAAGATCAG-3"). PCRs with these primers yielded a 679-bp product (22). Clindamycin MICs were not high for some erythromycin-resistant strains that were positive for the Erm(B) or the Erm(A) protein (3, 7, 23). These strains were checked for the presence of inducible erm by the erythromycin-clindamycin double-disk diffusion method, as described previously (5). Strains with the L4 mutation resembled mef organisms in that they were erythromycin resistant (with a narrower zone diameter than is usually the case with mef strains) and clindamycin susceptible.
Macrolide-resistant S. pneumoniae isolates that were negative for the erm and the mef genes were checked for the presence of mutations in ribosomal protein L4 or L22 or in 23S rRNA by using the primers and conditions described previously for S. pneumoniae (27). For 23S rRNA, specific oligonucleotide primers were used to amplify two portions of domain V of 23S rRNA; the first portion that includes nucleotides 2058 and 2059 was amplified with primers 5"-CGGCGGCCGTAACTATAACG-3" and 5"-GATGCGACGAGCCGACATCG-3" (nucleotides 1904 to 2522), and the second portion that nucleotide position 2611 was amplified with primers 5"-TCATTCGCAGAGTGTAAAGG-3" and 5"-TTGGATAAGTCCTCGAGCTATT-3" (nucleotides 2314 to 2902).
The PCR products were purified with a QIAquick PCR purification kit (Qiagen, Valencia, Calif.) and were sequenced with an Applied Biosystems model 373 DNA sequencer. Sequence comparisons were performed with Vector NTI sequence analysis software (Infomax, Inc., Bethesda, Md.). To rule out PCR contamination, L4 gene sequences were verified in independent experiments for 10 strains.
Determination of mechanisms of quinolone resistance.
For the pneumococcal strains for which levofloxacin MICs were ≥8 μg/ml, the sequences of topoisomerase IV (ParC, ParE) and DNA gyrase (GyrA, GyrB) were analyzed by PCR and DNA sequencing as described previously (19).
Serotyping of pneumococci and PFGE.
Serotyping of macrolide-resistant pneumococcal strains was performed by the standard Quellung method with antisera from Statens Seruminstitut (Copenhagen, Denmark). To determine the clonality of resistance in S. pneumoniae, pulsed-field gel electrophoresis (PFGE) with a CHEF DR III apparatus (Bio-Rad Laboratories, Hercules, Calif.) was performed as described previously (19). Interpretation of the interrelationships of PFGE patterns was performed by use of the criteria of Tenover et al. (28).
RESULTS
Age distributions and sources of isolates of S. pneumoniae
The age distributions of patients from whom S. pneumoniae isolates were recovered are shown in Table 1. In Hungary, Latvia, and the Slovak Republic, the age group with the highest rate of infection was children ages 2 to 10 years, while in the other countries the age group with the highest rate of infection was adults (21 to 60 or >61 years old).
TABLE 1.
Age distribution of patients infected with pneumococcal strains
| Country | No. of patients in the following age group (yr):
|
|||||
|---|---|---|---|---|---|---|
| <2 | 2-10 | 11-20 | 21-60 | >61 | Total | |
| Slovak Republic | 15 | 37 | 1 | 29 | 18 | 100 |
| Romania | 7 | 22 | 16 | 46 | 8 | 99 |
| Hungary | 26 | 35 | 3 | 22 | 16 | 102 |
| Lithuania | 5 | 24 | 9 | 36 | 19 | 93 |
| Slovenia | 2 | 30 | 4 | 30 | 35 | 101 |
| Czech Republic | 14 | 13 | 4 | 38 | 33 | 102 |
| Latvia | 9 | 58 | 29 | 0 | 0 | 96 |
| Bulgaria | 16 | 17 | 23 | 32 | 13 | 101 |
| Poland | 6 | 12 | 19 | 41 | 19 | 97 |
| Croatia | 11 | 9 | 4 | 36 | 41 | 101 |
The sources of clinical specimens of S. pneumoniae are shown in Table 2. Among the 992 pneumococcal isolates tested, 375 (37.8%) were isolated from sputum or tracheobrochial fluid, 212 (21.3%) were isolated from blood, 115 (11.6%) were isolated from the nasopharynx, and 89 (9.0%) were isolated from cerebrospinal fluid (CSF). The proportions of isolates from specimens such as blood and CSF from patients with systemic disease were 86.1% in Slovenia, 55.6% in Romania, 52.5% in Bulgaria, 42.2% in the Czech Republic, 23.0% in the Slovak Republic, 21.8% in Croatia, 9.8% in Hungary, 5.2% in Poland, and 3.2% in Lithuania. No pneumococcal strains were isolated from the CSF or blood of patients in Latvia.
TABLE 2.
Sources of pneumococcal isolates
| Country | No. (%) of specimens by source
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sputuma | Blood | CSF | Nasopharynx | Pleural | Sinusb | Eye | Ear | Other | Total | |
| Slovak Republic | 33 (33.0) | 15 (15.0) | 8 (8.0) | 0 | 4 (4.0) | 0 | 0 | 40 (40.0) | 0 | 100 |
| Romania | 31 (31.3) | 40 (40.4) | 15 (15.2) | 0 | 1 (1.0) | 0 | 3 (3.0) | 9 (9.1) | 0 | 99 |
| Hungary | 37 (36.3) | 8 (7.8) | 2 (2.0) | 19 (18.6) | 0 | 0 | 0 | 36 (35.3) | 0 | 102 |
| Lithuania | 49 (52.7) | 2 (2.2) | 1 (1.1) | 0 | 3 (3.2) | 28 (30.0) | 1 (1.1) | 7 (7.5) | 2c (2.2) | 93 |
| Slovenia | 7 (6.9) | 82 (81.1) | 5 (5.0) | 2 (2.0) | 3 (3.0) | 0 | 1 (1.0) | 1 (1.0) | 0 | 101 |
| Czech Republic | 31 (30.4) | 32 (31.3) | 11 (10.8) | 0 | 3 (2.9) | 2 (2.0) | 2 (2.0) | 21 (20.6) | 0 | 102 |
| Latvia | 2 (2.1) | 0 | 0 | 94 (97.9) | 0 | 0 | 0 | 0 | 0 | 96 |
| Bulgaria | 24 (23.7) | 11 (10.9) | 42 (41.6) | 0 | 12 (11.9) | 0 | 0 | 12 (11.9) | 0 | 101 |
| Poland | 92 (94.8) | 5 (5.2) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 97 |
| Croatia | 69 (68.3) | 17 (16.8) | 5 (4.9) | 0 | 2 (2.0) | 2 (2.0) | 2 (2.0) | 3 (3.0) | 1d (1.0) | 101 |
Including the tracheobronchial tract.
Maxillary sinus.
Peritoneal fluid.
Vaginal fluid.
MIC distributions for S. pneumoniae isolates
The MICs at which 50% of isolates are inhibited (MIC50) and the MIC90s for the antibacterial agents tested for the S. pneumoniae isolates recovered in the present study are shown in Table 3. The MIC90 of telithromycin was 0.06 μg/ml; and those of erythromycin, azithromycin, clarithromycin, and clindamycin were >64 μg/ml. The MIC90s of penicillin G and levofloxacin were both 2 μg/ml. The penicillin G MIC90s were the highest for the isolates from Hungary (8 μg/ml), followed by those for isolates from Romania and the Slovak Republic (4 μg/ml). The MIC90s of penicillin G (≥0.125 μg/ml) were in the nonsusceptible range for isolates from all countries except Latvia (0.03 μg/ml) and Lithuania (0.06 μg/ml). Clindamycin MIC90s were >64 μg/ml for isolates from Croatia, Hungary, Poland, Romania, and the Slovak Republic.
TABLE 3.
MIC50s and MIC90s for the S. pneumoniae isolates by agar dilution
| Country | MIC50/MIC90 (μg/ml)
|
||||||
|---|---|---|---|---|---|---|---|
| Penicillin G | Telithromycin | Erythromycin | Azithromycin | Clarithromycin | Clindamycin | Levofloxacin | |
| Slovak Republic | 0.25/4.0 | 0.016/0.125 | 0.06/>64.0 | 0.125/>64.0 | 0.06/>64.0 | 0.06/>64.0 | 1.0/2.0 |
| Romania | 0.125/4.0 | 0.016/0.06 | 0.06/>64.0 | 0.125/>64.0 | 0.03/>64.0 | 0.06/>64.0 | 2.0/2.0 |
| Hungary | 0.03/8.0 | 0.016/0.06 | 0.06/>64.0 | 0.125/>64.0 | 0.03/>64.0 | 0.06/>64.0 | 1.0/2.0 |
| Lithuania | 0.03/0.06 | 0.016/0.03 | 0.03/0.06 | 0.125/0.125 | 0.03/0.06 | 0.06/0.125 | 1.0/2.0 |
| Slovenia | 0.03/2.0 | 0.008/0.016 | 0.03/0.06 | 0.06/0.125 | 0.03/0.06 | 0.06/0.125 | 1.0/2.0 |
| Czech Republic | 0.03/2.0 | 0.016/0.03 | 0.06/0.06 | 0.125/0.25 | 0.03/0.06 | 0.06/0.25 | 2.0/4.0 |
| Latvia | 0.03/0.03 | 0.016/0.03 | 0.06/0.06 | 0.125/0.125 | 0.03/0.06 | 0.06/0.125 | 1.0/2.0 |
| Bulgaria | 0.03/2.0 | 0.016/0.06 | 0.06/8.0 | 0.125/8.0 | 0.03/4.0 | 0.06/0.125 | 1.0/2.0 |
| Poland | 0.03/0.25 | 0.016/0.06 | 0.06/>64.0 | 0.125/>64.0 | 0.03/>64.0 | 0.125/>64.0 | 1.0/2.0 |
| Croatia | 0.03/2.0 | 0.016/0.03 | 0.03/>64.0 | 0.06/>64.0 | 0.03/>64.0 | 0.06/>64.0 | 1.0/2.0 |
| All strains | 0.03/2.0 | 0.016/0.06 | 0.06/>64.0 | 0.125/>64.0 | 0.03/>64.0 | 0.06/>64.0 | 1.0/2.0 |
Comparative rates of drug susceptibility for the pneumococcal strains are listed in Table 4. Telithromycin susceptibility rates were 97.0 to 100% at a breakpoint of ≤0.5 μg/ml and 100% at a breakpoint of 2 μg/ml in each country (Table 5). The prevalence of penicillin G-intermediate (MICs, 0.125 to 1 μg/ml) pneumococcal isolates was 14.3%, and the prevalence of fully penicillin G-resistant (MICs, 2 to >16 μg/ml) isolates was 16.6%. The penicillin G susceptibility rates were lowest in Romania (44.5%) and the Slovak Republic (42.0%). Six strains from Croatia were fully resistant to levofloxacin (MICs, ≥8 μg/ml).
TABLE 4.
Rates of susceptibility to S. pneumoniae
| Country | % Susceptible
|
||||||
|---|---|---|---|---|---|---|---|
| Penicillin Ga | Telithromycinb | Erythromycin | Azithromycin | Clarithromycin | Clindamycin | Levofloxacin | |
| Slovak Republic | 21.0/37.0 | 98.0/100 | 72.0 | 72.0 | 72.0 | 89.0 | 100 |
| Romania | 25.2/30.3 | 97.0/100 | 67.6 | 67.6 | 67.6 | 73.7 | 97.9 |
| Hungary | 10.8/28.4 | 100/100 | 63.7 | 63.7 | 63.7 | 66.6 | 99.0 |
| Lithuania | 6.3/1.1 | 98.9/100 | 94.6 | 94.6 | 94.6 | 97.8 | 94.6 |
| Slovenia | 6.9/14.8 | 100/100 | 93.0 | 94.0 | 93.0 | 98.0 | 99.0 |
| Czech Republic | 8.8/10.7 | 97.1/100 | 95.1 | 95.1 | 95.1 | 96.1 | 75.5 |
| Latvia | 4.2/2.1 | 100/100 | 95.8 | 95.8 | 95.8 | 98.9 | 98.9 |
| Bulgaria | 21.8/17.8 | 100/100 | 79.2 | 80.1 | 80.1 | 94.0 | 90.0 |
| Poland | 16.5/4.1 | 100/100 | 72.1 | 72.1 | 72.1 | 77.3 | 98.9 |
| Croatia | 20.8/17.8 | 100/100 | 81.1 | 81.1 | 81.1 | 84.1 | 94.0 |
| Total | 14.3/16.6 | 99.8/100 | 81.3 | 81.5 | 81.7 | 87.5 | 94.7 |
For penicillin G, the values are for penicillin-intermediate (MICs, 0.125 to 1 μg/ml)/penicillin-resistant (MICs, ≥2 μg/ml) strains.
For telithromycin, the values are for breakpoints for telithromycin susceptibility at ≤0.5 μg/ml/≤2.0 μg/ml.
TABLE 5.
Incidence of macrolide resistance and mechanisms of resistance of isolates from 10 centers
| Country | No. (%) of macrolide-resistant S. pneumoniae strains | No. of strains with the following mechanism of resistance:
|
|||
|---|---|---|---|---|---|
| Erm(B) | Erm(A) | Mef(E) | L4a | ||
| Slovak Republic | 28 (28) | 11 | 0 | 0 | 17 |
| Romania | 31 (31.3) | 26 | 0 | 0 | 5 |
| Hungary | 36 (35.3) | 35 | 1 | 0 | 0 |
| Lithuania | 5 (5.4) | 2 | 0 | 3 | 0 |
| Slovenia | 5 (4.9) | 2 | 0 | 2 | 1 |
| Czech Republic | 5 (4.9) | 4 | 0 | 1 | 0 |
| Latvia | 4 (4.2) | 1 | 0 | 1 | 2 |
| Bulgaria | 20 (19.8) | 6 | 0 | 12 | 2 |
| Poland | 27 (27.8) | 22 | 1 | 3 | 1 |
| Croatia | 19 (18.8) | 16 | 0 | 3 | 0 |
| Total | 180 (18.1) | 125 | 2 | 25 | 28 |
Only strains negative for erm and mef were checked for the mutation in L4.
Macrolide resistance mechanism in S. pneumoniae
The prevalence of macrolide-resistant strains and the macrolide resistance mechanisms in each country are shown in Table 5. One hundred eighty pneumococcal isolates (18.1%) were erythromycin resistant (MIC >0.25 μg/ml). The prevalence of macrolide resistance was less than 10% in the Czech Republic (4.9%), Latvia (4.2%), Lithuania (5.4%), and Slovenia (4.9%), while the prevalence was 18.8 to 35.3% in the other countries. The erm(B) gene was found in 125 (69.4%) of the 180 erythromycin-resistant strains, the mef(E) gene was found in 25 (13.9%) of the 180 erythromycin-resistant strains, and 2 strains from Hungary and Poland had the erm(A) gene. Twelve strains with the mef(E) gene were found in Bulgaria (60%; 95% confidence interval, 36.1 to 80.9%); 3 strains with the mef(E) gene were found in Croatia, Lithuania, and Poland each; 2 strains with the mef(E) gene were found in Slovenia; and 1 strain with the mef(E) gene was found in the Czech Republic and Latvia each.
The correlation between the MIC distribution and the mechanism of resistance in S. pneumoniae is shown in Table 6. The MIC50s and the MIC90s of erythromycin, azithromycin, clarithromycin, and clindamycin for pneumococcal strains that had erm(B) genes were >64 and >64 μg/ml, respectively, while the MIC50 and the MIC90 of telithromycin were 0.03 and 0.5 μg/ml, respectively. For strains with mef(E), the MIC90s of erythromycin, azithromycin, and clarithromycin were 4 to 8 μg/ml and the MIC90s of telithromycin and clindamycin were 0.25 and 0.125 μg/ml, respectively. Two strains with erm(A) genes had inducible resistance to macrolides; and the telithromycin MICs were 0.03 and 0.125 μg/ml, respectively, the erythromycin MICs were 2 and 32 μg/ml, respectively, the azithromycin MICs were 4 and >64 μg/ml, respectively, the clarithromycin MICs were 0.06 and 1 μg/ml, respectively, and the clindamycin MICs were 0.06 and 1 μg/ml, respectively. For strains containing L4 mutations, the MIC90s of erythromycin, clarithromycin, and azithromycin were 64 to >64 μg/ml and the MIC90s of telithromycin and clindamycin were 0.25 and 0.125 μg/ml, respectively.
TABLE 6.
MIC distribution by mechanism of resistance in S. pneumoniae
| Drug and resistance mechanisma | MIC (μg/ml)
|
||
|---|---|---|---|
| Range | 50% | 90% | |
| Telithromycin | |||
| erm(B)C (n = 122) | 0.004-2 | 0.03 | 0.5 |
| erm(B)I (n = 3) | 0.004-0.015 | ||
| erm(A)I (n = 2) | 0.03-0.125 | ||
| mef(E) (n = 25) | 0.03-0.5 | 0.125 | 0.25 |
| L4 (n = 28) | 0.03-0.25 | 0.125 | 0.25 |
| Total (n = 180) | 0.004-2 | 0.06 | 0.25 |
| Azithromycin | |||
| erm(B)C (n = 122) | 16->64 | >64 | >64 |
| erm(B)I (n = 3) | 8->64 | ||
| erm(A)I (n = 2) | 4->64 | ||
| mef(E) (n = 25) | 1-16 | 4 | 8 |
| L4 (n = 28) | 1->64 | >64 | >64 |
| Total (n = 180) | 1->64 | >64 | >64 |
| Erythromycin | |||
| erm(B)C (n = 122) | 4->64 | >64 | >64 |
| erm(B)I (n = 3) | 2->64 | ||
| erm(A)I (n = 2) | 2-32 | ||
| mef(E) (n = 25) | 1-8 | 4 | 8 |
| L4 (n = 28) | 0.5->64 | >64 | >64 |
| Total (n = 180) | 0.5->64 | >64 | >64 |
| Clarithromycin | |||
| erm(B)C (n = 122) | 1->64 | >64 | >64 |
| erm(B)I (n = 3) | 1->64 | ||
| erm(A)I (n = 2) | 0.06-1 | ||
| mef(E) (n = 25) | 0.5-4 | 2 | 4 |
| L4 (n = 28) | 0.25->64 | 32 | 64 |
| Total (n = 180) | 0.25->64 | >64 | >64 |
| Clindamycin | |||
| erm(B)C (n = 122) | 1->64 | >64 | >64 |
| erm(B)I (n = 3) | 0.125-0.25 | ||
| erm(A)I (n = 2) | 0.06-1 | ||
| mef(E) (n = 25) | 0.03-0.125 | 0.06 | 0.125 |
| L4 (n = 28) | 0.03-0.125 | 0.125 | 0.125 |
| Total (n = 180) | 0.03->64 | >64 | >64 |
I, inducible; C, constitutive.
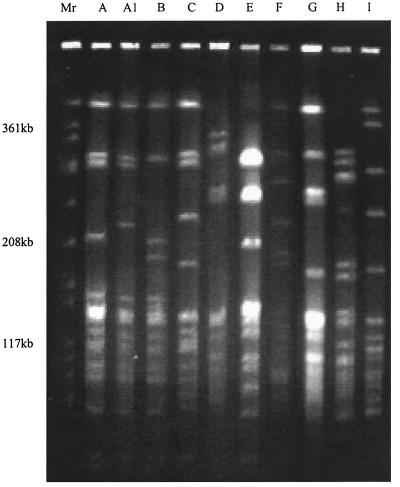
One L4 protein mutation consisted of a three-amino-acid alteration of 69GlyTyrGly to TyrPheSer and was found in 27 (15.0%) of the 180 erythromycin-resistant strains (Table 7). Seventeen of the 27 strains (63.0%) with the L4 mutation were from the Slovak Republic, 5 strains were from Romania, 2 strains each were from Bulgaria and Latvia, and 1 strain was from Poland. The MICs of erythromycin (>64 μg/ml) and azithromycin (>64 μg/ml) were higher for most strains, but all except one strain from Slovenia (Tables 6 and 7) were susceptible to clindamycin (MICs, 0.06 to 0.125) and telithromycin (MICs, 0.03 to 0.25). The strain with the L4 protein mutation from Slovenia had an amino acid change different from those for the other strains that comprised a three-amino-acid insertion (ArgArgGln) between 67Arg and 68Lys in the L4 proteins of the pneumococci. The PFGE patterns showed that two strains from Bulgaria were similar to a strain from Latvia and the Slovak Republic (PFGE type C); strains from the Slovak Republic had very similar PFGE patterns (PFGE types A, A1, and B) (Fig. 1).However, strains from Romania (PFGE types D, G, and H) and one strain each from Latvia (PFGE type I), Slovenia (PFGE type E), and Poland (PFGE type F) had PFGE types different from those of the other strains tested. The serotypes of strains with the L4 protein mutation from the Slovak Republic, Bulgaria, and Latvia were 19A and 19F; the strain from Slovenia was type 5, and the strain from Poland was 23F. Only one Romanian strain was typeable with pooled sera and was found to be type 9V. Forty-one (22.8%) of the 180 macrolide-resistant strains were penicillin G susceptible (MICs, ≤0.06 μg/ml), 64 (35.5%) strains were penicillin G intermediate (MICs, 0.125 to 1 μg/ml), and 75 (41.7%) strains were penicillin G resistant (MICs, ≥2 μg/ml) (Table 7).
TABLE 7.
Profiles of macrolide-resistant S. pneumoniae with L4 protein mutations
| Strain no. | Country | Source | Serotype | L4 Mutation | PFGE pattern | % incidence (all strains/resistant strains) |
|---|---|---|---|---|---|---|
| 1384 | Slovak Republic | Ear fluid | 19A | 69GTGa71 to TPSb | A | |
| 1386 | Slovak Republic | Ear fluid | 19A | 69GTG71 to TPS | A | |
| 1394 | Slovak Republic | Ear fluid | 19A | 69GTG71 to TPS | A | |
| 1397 | Slovak Republic | Ear fluid | 19A | 69GTG71 to TPS | A1 | |
| 1407 | Slovak Republic | Ear fluid | 19A | 69GTG71 to TPS | A | |
| 1409 | Slovak Republic | Sputum | 19A | 69GTG71 to TPS | A1 | |
| 1424 | Slovak Republic | Sputum | 19A | 69GTG71 to TPS | A | |
| 2476 | Slovak Republic | Sputum | 19A | 69GTG71 to TPS | B | |
| 2478 | Slovak Republic | Sputum | 19F | 69GTG71 to TPS | A | 17.0% (n = 100)/60.7% (n = 28) |
| 2487 | Slovak Republic | Ear fluid | 19A | 69GTG71 to TPS | A | |
| 2506 | Slovak Republic | Blood | 19A | 69GTG71 to TPS | A | |
| 2508 | Slovak Republic | CSF | 19A | 69GTG71 to TPS | A1 | |
| 2509 | Slovak Republic | Blood | 19F | 69GTG71 to TPS | B | |
| 2514 | Slovak Republic | Lung | 19A | 69GTG71 to TPS | A1 | |
| 2517 | Slovak Republic | Blood | 19A | 69GTG71 to TPS | B | |
| 2519 | Slovak Republic | Sputum | 19F | 69GTG71 to TPS | C | |
| 2526 | Slovak Republic | CSF | 19F | 69GTG71 to TPS | A1 | |
| 1653 | Bulgaria | Ear fluid | 19A | 69GTG71 to TPS | C | 2.0% (n = 101)/10.0% (n = 20) |
| 1663 | Bulgaria | Ear fluid | 19A | 69GTG71 to TPS | C | |
| 1541 | Romania | Ear fluid | NTc | 69GTG71 to TPS | D | 5.1% (n = 99)/16.1% (n = 31) |
| 1548 | Romania | Ear fluid | NT | 69GTG71 to TPS | D | |
| 1564 | Romania | Ear fluid | NT | 69GTG71 to TPS | D | |
| 1487 | Romania | Blood | NT | 69GTG71 to TPS | G | |
| 1495 | Romania | Sputum | 9V | 69GTG71 to TPS | H | |
| 2224 | Latvia | BALd | 19A | 69GTG71 to TPS | C | 2.1% (n = 96)/50.0% (n = 4) |
| 2249 | Latvia | Throat | 19F | 69GTG71 to TPS | I | |
| 2378 | Slovenia | Blood | 5 | 67Q-RRQ-K68 | E | 1.0% (n = 101)/20.0% (n = 5) |
| 1815 | Poland | Sputum | 23F | 69GTG71 to TPS | F | 1.0% (n = 97)/3.7% (n = 27) |
| Total | 2.8% (n = 992)/15.6% (n = 180) |
GlyTyrGly.
TPS, TyrPheSer.
NT, not typeable.
BAL, bronchoalveolar lavage fluid.
FIG. 1.
Examples of each PFGE pattern of erythromycin-resistant S. pneumoniae isolates with the L4 protein mutation. The PFGE pattern is indicated at the top of each lane. Lane Mr, S. aureus ATCC 8325 was used as the molecular size standard.
Distribution of serotypes in macrolide-resistant S. pneumoniae
The predominant serotypes in erythromycin-resistant S. pneumoniae isolates were type 6B (35 strains [19.4%]), types 14 and 19F (28 strains each [15.6% each]), type 19A (24 strains [13.3%]), and type 23F (19 strains [10.6%]). In each country, the predominant serotypes among the erythromycin-resistant strains were type 6A in Romania (11 strains [35.5%]); type 6B in Poland (13 strains [48.2%]); type 14 in Bulgaria (6 strains [30.0%]) and the Czech Republic (4 strains [80.0%]); type 19F in Croatia (5 strains [26.3%]), Latvia (2 strains [50%]), and Slovenia (2 strains [40.0%]); type 19A in Hungary (10 strains [27.8%]) and the Slovak Republic (12 strains [42.9%]); and type 23F in Lithuania (4 strains [80.0%]).
Pneumococci highly resistant to levofloxacin in some countries.
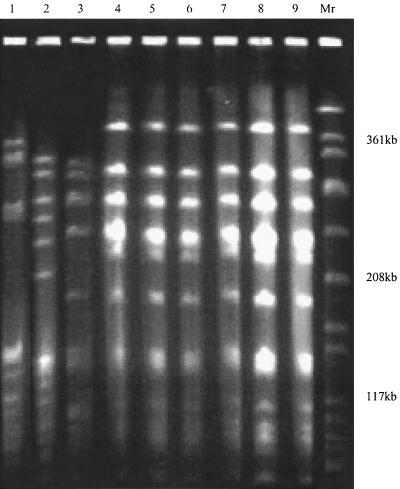
Among all S. pneumoniae isolates tested, levofloxacin MICs were high for nine strains (0.9%) (>8 μg/ml). Six of the nine levofloxacin-resistant strains were from Croatia; and one strain each was from the Czech Republic, Poland, and Romania. The isolate from Romania also had an L4 protein mutation and had high-level penicillin G resistance (MIC, 4 μg/ml). All strains were found to have amino acid substitutions in both ParC (Ser79 to Phe) and GyrA (Ser81 to Tyr or Phe or Glu85 to Lys) of the quinolone resistance-determining region, but not in ParE and GyrB, when their sequences were compared to the wild-type sequence. Isolates from Croatia had the same PFGE patterns, but their PFGE patterns did not match the PFGE patterns of levofloxacin-resistant isolates from the other countries (Fig. 2).All Croatian strains were serotype 23F, and the strain from Poland was serotype 14. Strains from the Czech Republic and Romania were not typeable with pooled sera.
FIG. 2.
PFGE patterns of S. pneumoniae isolates with high-level levofloxacin resistance. Lane 1, strain 1541 from Romania; lane 2, strain 1813 from Poland; lane 3, strain 2065 from the Czech Republic; lanes 4 to 9, strains 2527, 2529, 2536, 2538, 2542, and 2578 from Croatia, respectively; lane Mr, S. aureus ATCC 8325 was used as the molecular size standard.
DISCUSSION
In the present study, the overall rate of macrolide resistance among the S. pneumoniae isolates tested was 18.1%; however, the prevalence of macrolide resistance showed considerable variation (4.2 to 35.3%). Macrolide resistance in S. pneumoniae correlated with penicillin resistance in a majority of strains; however, 41 macrolide-resistant strains (22.8%) were penicillin G susceptible. These strains were found in all countries except Romania. This is different from the resistance pattern found in the United States, where most erythromycin-resistant strains are cross resistant to penicillin G (14).
The most prevalent pneumococcal macrolide resistance mechanism in the present study was erm(B). Three strains containing the erm(B) gene had inducible resistance, as determined by the double-disk test (5). Inducible resistance in S. pneumoniae is rare but has been also reported in Belgium (9). We found two strains which had the erm(A) gene. The two strains differed in their drug susceptibility profiles and PFGE patterns and were also different from the pneumococcal erm(A) clone isolated from Greece reported by Syrogiannopoulos et al. (25).
In some countries such as the Czech Republic, Latvia, Lithuania, and Slovenia, the prevalence of erythromycin-resistant strains was lower; a possible reason for this might be limited antibacterial use in those countries. In Lithuania and Latvia, where the rates of penicillin G and erythromycin resistance in S. pneumoniae are still very low, the use of penicillin G may still be recommended. In Latvia and Lithuania, pneumococcal infections are treated with penicillin G, ampicillin, or amoxicillin (the last two are used with or without β-lactamase inhibitors) (L. S. Stratchounski, personal communication, and L. Drukalska, unpublished data). We hypothesize that possible causes for the higher prevalence of erythromycin-resistant S. pneumoniae in the other countries could be the abuse of macrolides or the clonal spread of resistant clones (4). We are not aware of other reports on this subject, which is under investigation. We examined the PFGE patterns of pneumococcal isolates with L4 protein mutations and found some clones that appear to be circulating within the same country and spreading to neighboring countries. In the case of S. pneumoniae, an L4 protein mutation was first described in laboratory mutant strains selected with azithromycin (27) and was then reported among 16 clinical isolates from Eastern European countries that contained a three-amino-acid substitution (69GTG71 to TPS) (26). This is the first report that provides data on the prevalence of this type of mechanism of resistance among erythromycin-resistant S. pneumoniae strains. For one strain from Slovenia, which had an insertion of three amino acids between 67Arg and 68Lys, erythromycin MICs were lower compared to those for other strains with different L4 mutations. This type of L4 protein mutation has not previously been described in a clinical isolate.
Levofloxacin-resistant S. pneumoniae isolates from Croatia also had the same PFGE patterns as those of the isolates from the Czech Republic, Latvia, Lithuania, and Slovenia, as described above. Investigations are being conducted to compare this clone and the other multidrug-resistant clone of type 23F. The possible clonal spread of this clone to other countries is a cause for concern because of the increasing use of broad-spectrum fluoroquinolones such as levofloxacin, gatifloxacin, and moxifloxacin.
A pneumococcal strain from Romania was resistant to penicillin G, macrolides, and fluoroquinolones. Multidrug-resistant strains such as this clone represent a potentially serious therapeutic problem. Fluoroquinolone-resistant S. pneumoniae isolates have recently been reported in Canada (6) and Spain (18), and multiple-drug-resistant strains have been reported from Hong Kong (13). Careful monitoring and surveillance will be important to see if these fluoroquinolone-resistant and multidrug-resistant strains will spread to other countries or continents. Broad-spectrum fluoroquinolones should be used judiciously to prevent resistance, and serious thought should be given before their widespread introduction into the pediatric group.
The rates of susceptibility to telithromycin among the S. pneumoniae isolates tested were higher than those to the macrolides. Irrespective of the mechanism of macrolide resistance, telithromycin was active. The MIC50 and the MIC90 of telithromycin for erythromycin-resistant S. pneumoniae isolates were 0.06 and 0.25 μg/ml, respectively, and the rate of susceptibility to telithromycin at ≤0.5 μg/ml was 99.8% among all S. pneumoniae isolates tested. All strains were susceptible to telithromycin at ≤2.0 μg/ml.
We realize that the heterogeneity of the clinical samples tested (Table 2) precludes an accurate comparison of resistance rates. However, with the data at hand (which require confirmation with strains isolated from similar sources in each center), telithromycin had excellent in vitro activity against S. pneumoniae, including drug-resistant strains. The rate of penicillin resistance in S. pneumoniae was the highest in the Slovak Republic and Romania, and erythromycin resistance was most frequently found among isolates from Hungary and Romania. Fifteen percent of erythromycin-resistant S. pneumoniae isolates were found to have L4 protein mutations. Our findings point to the potential use of telithromycin in the treatment of infections caused by macrolide-susceptible and -resistant pneumococci.
Acknowledgments
This study was supported by a grant from Aventis, Romainville, France.
REFERENCES
- 1.Appelbaum, P. C. 1992. Antimicrobial resistance in Streptococcus pneumoniae: an overview. Clin. Infect. Dis. 15:77-83. [DOI] [PubMed] [Google Scholar]
- 2.Appelbaum, P. C., C. Gladkova, W. Hryniewicz, B. Kojouharov, D. Kotulova, F. Mihalcu, J. Schindler, L. Setchanova, N. Semina, J. Trupl, S. Tyski, P. Urbaskova, and M. R. Jacobs. 1996. Carriage of antibiotic-resistant Streptococcus pneumoniae by children in Eastern and Central Europe--a multicenter study with use of standardized methods. Clin. Infect. Dis. 23:712-717. [DOI] [PubMed] [Google Scholar]
- 3.Arthur, M., C. Molinas, C. Mabilat, and P. Courvalin. 1990. Detection of erythromycin resistance by the polymerase chain reaction in conserved regions of erm rRNA methylase genes. Antimicrob. Agents Chemother. 34:2024-2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baquero, F. 1996. Trends in antibiotic resistance of respiratory pathogens: an analysis and commentary on a collaborative surveillance study. J. Antimicrob. Chemother. 38(Suppl. A):117-132. [DOI] [PubMed] [Google Scholar]
- 5.Bemer-Melchior, P., M. E. Juvin, S. Tassin, A. Bryskier, G. C. Schito, and H. B. Drugeon. 2000. In vitro activity of the new ketolide telithromycin compared with those of macrolides against Streptococcus pyogenes: influence of resistance mechanisms and methodological factors. Antimicrob. Agents Chemother. 44:2999-3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, D. K., A. McGeer, J. C. De Azavedo, and D. E. Low. 1999. Decreased susceptibility of Streptococcus pneumoniae to fluoroquinolones in Canada. N. Engl. J. Med. 341:233-239. [DOI] [PubMed] [Google Scholar]
- 7.Clancy, J., J. Petitpas, F. Dib-Hajj, W. Yuan, M. Cronan, A. V. Kamath, J. Bergeron, and J. A. Retsema. 1996. Molecular cloning and functional analysis of a novel macrolide resistance determinant, mefA, from Streptococcus pyogenes. Mol. Microbiol. 22:867-879. [DOI] [PubMed] [Google Scholar]
- 8.Davies, T. A., L. M. Kelly, M. R. Jacobs, and P. C. Appelbaum. 2000. Antipneumococcal activity of telithromycin by agar dilution, microdilution, E-test and disk diffusion. J. Clin. Microbiol. 38:1444-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Descheemaeker, P., S. Chapelle, C. Lammens, M. Hauchecorne, M. Wijdooghe, P. Vandamme, M. Ieven, and H. Goossens. 2000. Macrolide resistance and erythromycin resistance determinants among Belgian Streptococcus pneumoniae isolates. J. Antimicrob. Chemother. 45:167-173. [DOI] [PubMed] [Google Scholar]
- 10.Ednie, L., S. K. Spangler, M. R. Jacobs, and P. C. Appelbaum. 1997. Susceptibilities of 228 penicillin-and erythromycin-susceptible and -resistant pneumococci to RU 64004, a new ketolide, compared with susceptibilities to 16 other agents. Antimicrob. Agents Chemother. 41:1033-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fasola, E. L., S. Bajaksouzian, P. C. Appelbaum, and M. R. Jacobs. 1997. Variation in erythromycin and clindamycin susceptibilities of Streptococcus pneumoniae by four test methods. Antimicrob. Agents Chemother. 41:129-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Felmingham, D., R. N. Grünenburg, and the Alexander Project Group. 2000. The Alexander Project 1996-1997: last susceptibility data from this international study of bacterial pathogens from community-acquired lower respiratory tract infections. J. Antimicrob Chemother. 45:191-203. [DOI] [PubMed] [Google Scholar]
- 13.Ho, P. L., T. L. Que, D. N. C. Tsang, T. K. Ng, K. H. Chow, and W. H. Seto. 1999. Emergence of fluoroquinolone resistance among multiply resistant strains of Streptococcus pneumoniae in Hong Kong. Antimicrob. Agents Chemother. 43:1310-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobs, M. R., S. Bajaksouzian, A. Zilles, G. Lin, G. A. Pankuch, and P. C. Appelbaum. 1999. Susceptibilities of Streptococcus pneumoniae and Haemophilus influenzae to 10 oral antimicrobial agents based on pharmacodynamic parameters: 1997 U.S. surveillance study. Antimicrob. Agents Chemother. 43:1901-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jamjian, C., D. J. Biedenbach, and R. N. Jones. 1997. In vitro evaluation of a novel ketolide antimicrobial agent, RU-64004. Antimicrob. Agents Chemother. 41:135-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones, R. N., and D. J. Biedenbach. 1997. Antimicrobial activity of RU-66647, a new ketolide. Diagn. Microbiol. Infect. Dis. 27:7-12. [DOI] [PubMed] [Google Scholar]
- 17.Leclercq, R., and P. Courvalin. 1991. Bacterial resistance to macrolide, lincosamide, and streptogramin antibiotics by target modification. Antimicrob. Agents Chemother. 35:1267-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liñares, J., A. G. de la Campa, and R. Pallares. 1999. Fluoroquinolone resistance in Streptococcus pneumoniae. N. Engl. J. Med. 341:1546-1547. [DOI] [PubMed] [Google Scholar]
- 19.Nagai, K., T. A. Davies, G. A. Pankuch, B. E. Dewasse, and P. C. Appelbaum. 2000. Resistance to clinafloxacin, ciprofloxacin, and trovafloxacin in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 44:2740-2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Committee for Clinical Laboratory Standards. 1997. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 4th ed. Approved standard. NCCLS document M7-A4. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 21.Pankuch, G. A., M. A. Visalli, M. R. Jacobs, and P. C. Appelbaum. 1998. Susceptibilities of penicillin- and erythromycin-susceptible and -resistant pneumococci to HMR 3647 (RU 66647), a new ketolide, compared with susceptibilities to 17 other agents. Antimicrob. Agents Chemother. 42:624-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seppälä, H., M. Skurnik, H. Soini, M. C. Roberts, and P. Huovinen. 1998. A novel erythromycin resistance methylase gene (ermTR) in Streptococcus pyogenes. Antimicrob. Agents Chemother. 42:257-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sutcliffe, J., T. Grebe, A. Tait-Kamradt, and L. Wondrack. 1996. Detection of erythromycin-resistant determinants by PCR. Antimicrob. Agents Chemother. 40:2562-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sutcliffe, J., A. Tait-Kamradt, and L. Wondrack. 1996. Streptococcus pneumoniae and Streptococcus pyogenes resistant to macrolides but sensitive to clindamycin: a common resistance pattern by an efflux system. Antimicrob. Agents Chemother. 40:1817-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Syrogiannopoulos, G. A., I. N. Grivea, A. Tait-Kamradt, G. D. Katopodis, N. G. Beratis, J. Sutcliffe, P. C. Appelbaum, and T. A. Davies. 2001. Identification of an erm(A) erythromycin resistance methylase gene in Streptococcus pneumoniae isolated in Greece. Antimicrob. Agents Chemother. 45:342-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tait-Kamradt, A., T. Davies, P. C. Appelbaum, F. Depardieu, P. Courvalin, J. Petitpas, L. Wondrack, A. Walker, M. R. Jacobs, and J. Sutcliffe. 2000. Two new mechanisms of macrolide resistance in clinical strains of Streptococcus pneumoniae from Eastern Europe and North America. Antimicrob. Agents Chemother. 44:3395-3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tait-Kamradt, A., T. Davies, M. Cronan, M. R. Jacobs, P. C. Appelbaum, and J. Sutcliffe. 2000. Mutation in 23S rRNA and ribosomal protein L4 account for resistance in pneumococcal strains selected in vitro by macrolide passage. Antimicrob. Agents Chemother. 44:1894-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tenover, F. C., R. D. Arberit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulse-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2223-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weisblum, B. 1995. Erythromycin resistance by ribosome modification. Antimicrob. Agents Chemother. 39:577-585. [DOI] [PMC free article] [PubMed] [Google Scholar]