Abstract
Pseudomonas aeruginosa clinical isolate CY-1, which was resistant to ceftazidime, harbored a conjugative ca. 250-kb plasmid that contained a class 1 integron with two gene cassettes encoding OXA-32, an OXA-2- type β-lactamase, and the aminoglycoside acetyltransferase AAC(6′)Ib9. OXA-32 differed from OXA-2 by an Leu169Ile amino acid substitution (class D numbering). Site-directed mutagenesis established that Ile169 is responsible for resistance to ceftazidime but not to cefotaxime.
The OXA enzymes belong to Ambler class D and possess an active serine site similar to class A and class C β-lactamases (7). The OXA enzymes are mostly narrow-spectrum β-lactamases (OXA-1 to OXA-5, OXA-6, OXA-7, OXA-9, OXA-10, OXA-11, OXA-13, OXA-20 to OXA-22, and LCR-1) (12). They usually confer resistance to amino- and carbenipenicillins and narrow-spectrum cephalosporins (12). Extended-spectrum OXA enzymes capable of hydrolyzing ceftazidime, cefotaxime, or aztreonam include the OXA-10 derivatives (OXA-11, OXA-14, OXA-16, OXA-17, OXA-19, and OXA-28) (12, 17) clavulanic-acid susceptible enzyme OXA-18 (16), and OXA-15, the only known extended-spectrum derivative of OXA-2 (4). Oxacillin-hydrolyzing (OXA) enzymes are frequently found in Pseudomonas aeruginosa (12, 15). Most of the extended-spectrum OXA enzymes including OXA-15 have been reported in P. aeruginosa isolates from Turkey (13), whereas two carbapenem-hydrolyzing OXA enzymes, OXA-24 and ARI-1 (OXA-23), have reported recently been from Spain and the United Kingdom, respectively (1, 5). The oxa genes are the β-lactamase genes that are most often part of gene cassettes located in class 1 integrons (12, 14). Integrons are DNA structures capable of capturing gene cassettes that usually correspond to antibiotic resistance gene cassettes (6, 19).
P. aeruginosa CY-1 was resistant to ceftazidime and had wild-type and low-level resistance to cefotaxime on the basis of antibiotic susceptibility testing by disk diffusion. It was isolated in 1996 from a culture of skin from a hospitalized patient on the day of his admission to the intensive care unit of the Hôpital d’Instruction des Armées Percy (Clamart, France) for cure of skin burns. He had been transferred from the intensive care unit of the university hospital of Pointe-à-Pitre (Guadeloupe, French West Indies), where he had previously been hospitalized for 7 consecutive days. Thus, P. aeruginosa CY-1 was considered a nosocomial isolate from the latter hospital.
P. aeruginosa CY-1 was identified with the API 20NE system (bioMérieux, Marcy-l’Etoile, France). The MICs of β-lactams were determined by an agar dilution method on Mueller-Hinton agar (Sanofi-Diagnostics Pasteur), as described previously (16). P. aeruginosa CY-1 was resistant to ceftazidime and had reduced susceptibilities to ticarcillin, cefepime, aztreonam, and imipenem (Table 1). The addition of clavulanic acid slightly decreased the ticarcillin MIC (Table 1). Antibiotic susceptibility testing by disk diffusion showed that P. aeruginosa CY-1 was also resistant to all clinically available aminoglycosides, tetracycline, and nalidixic acid but was susceptible to ciprofloxacin (data not shown).
TABLE 1.
MICs of β-lactams for clinical isolate P. aeruginosa CY-1, P. aeruginosa PU21 harboring natural plasmid pGER-1, reference strain P. aeruginosa PU21, E. coli DH10B harboring recombinant plasmids pPG13 and pPG14, and reference strain E. coli DH10B
| β-Lactam(s)a | MIC (μg/ml)
|
|||||
|---|---|---|---|---|---|---|
| P. aeruginosa CY-1 (OXA-32) | P. aeruginosa PU21(pGER-1)(OXA-32) | P. aeruginosa PU21 | E. coli DH10B(pPG13) (OXA-32) | E. coli DH10B(pPG14) (OXA-2) | E. coli DH10B | |
| Amoxicillin | >512 | >512 | >512 | 256 | >512 | 4 |
| Amoxicillin + CLA | >512 | >512 | >512 | 16 | 64 | 4 |
| Ticarcillin | 64 | 256 | 8 | 128 | >512 | 4 |
| Ticarcillin + CLA | 32 | 64 | 4 | 16 | 32 | 4 |
| Piperacillin | 16 | 32 | 8 | 64 | 64 | 1 |
| Piperacillin + TZB | 4 | 16 | 16 | 8 | 2 | 1 |
| Cephalothin | >512 | >512 | >512 | 16 | 64 | 2 |
| Cefsulodin | 32 | 32 | 2 | 16 | 16 | 2 |
| Ceftazidime | 128 | 128 | 1 | 32 | 4 | 0.5 |
| Ceftazidime + CLA | 128 | 128 | 8 | 16 | 0.5 | 0.5 |
| Cefotaxime | 8 | 16 | 8 | 0.5 | 0.12 | 0.06 |
| Cefepime | 8 | 8 | 1 | 0.25 | 0.06 | 0.06 |
| Cefpirome | 32 | 16 | 4 | 0.5 | 0.06 | 0.06 |
| Moxalactam | 32 | 64 | 8 | 0.5 | 0.12 | 0.12 |
| Aztreonam | 16 | 32 | 4 | 2 | 0.12 | 0.12 |
| Imipenem | 8 | 4 | 2 | 0.12 | 0.12 | 0.12 |
CLA, clavulanic acid at a fixed concentration of 2 μg/ml; TZB, tazobactam at a fixed concentration of 4 μg/ml.
A β-lactamase extract of a 10-ml culture of P. aeruginosa CY-1 was submitted to analytical isoelectric focusing (IEF) on an ampholin polyacrylamide gel, as described previously (17). It gave bands for two β-lactamases with pI values of 7.7 and 8.5. The band with the pI value of 8.5 likely corresponded to the chromosomal AmpC cephalosporinase of P. aeruginosa (data not shown). Whole-cell DNA of P. aeruginosa CY-1 was extracted as described previously (16). Fragments of BamHI-digested whole-cell DNA were cloned into the pBK-CMV phagemid (Stratagene, Amsterdam, The Netherlands) and expressed in Escherichia coli DH10B, as described previously (16). Antibiotic-resistant colonies were selected on ceftazidime (4 μg/ml)- and kanamycin (30 μg/ml)-containing Trypticase soy agar plates. A recombinant plasmid, pPG13, was retained for further analysis.
The MICs of the β-lactams for E. coli DH10B(pPG13) showed that it was resistant to amoxicillin, ticarcillin, and ceftazidime, had reduced susceptibilities to piperacillin and cephalothin, and remained susceptible to cefotaxime and aztreonam (Table 1). The addition of clavulanic acid to ceftazidime has little effect on the MIC of the latter (Table 1). IEF analysis of the β-lactamase extracted from a 100-ml culture of E. coli DH10B(pPG13) gave a single band with a pI value of 7.7, as was found in P. aeruginosa CY-1 (data not shown).
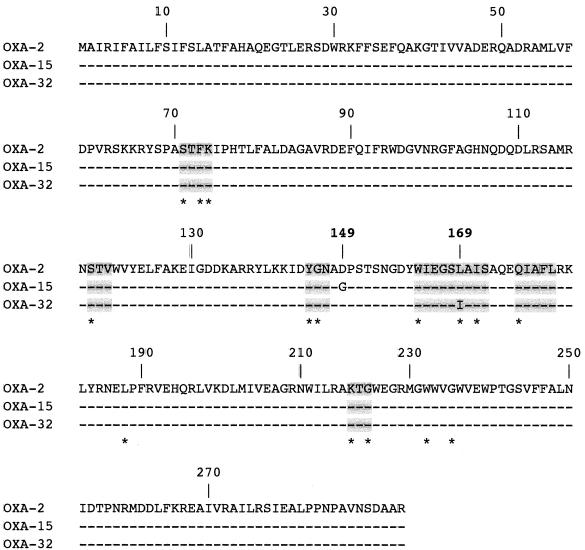
Both strands of the 4,700-bp cloned DNA fragment from plasmid pPG13 were partially sequenced, and the sequence was analyzed as reported previously (17). It encoded two open reading frames (ORFs) for antibiotic resistance genes. The first ORF, of 728 bp, encoded a 275-amino-acid preprotein, named OXA-32. Compared to the OXA-2 β-lactamase (3), OXA-32 has a Leu-to-Ile substitution at position DBL 169 (class D β-lactamase numbering [2]) (Fig. 1). This substitution was located in a conserved element of class D β-lactamases and corresponded to a highly conserved residue (12) (Fig. 1). The extended-spectrum properties of OXA-15 compared to those of OXA-2 have been related to the Asn149Gly substitution next to the YGN conserved motif of class D β-lactamases, a substitution that is not found in OXA-32 (Fig. 1).
FIG. 1.
Comparison of the amino acid sequence of OXA-32 to those of OXA-15 and OXA-2. Dashes indicate identical amino acids. The numbering is according to the class D β-lactamase (DBL) numbering (2). The numbers of the amino acid positions involved in the extended-spectrum profile of either OXA-32 or OXA-15 are in boldface. The highlighted boxes indicate conserved regions within class D β-lactamases, and highly conserved residues are shown by asterisks.
To further characterize the activity of OXA-32, it was extracted from 4 liters of a culture of E. coli DH10B(pPG13) as reported previously (17). A 30-ml supernatant containing the enzyme in 20 mM Tris-H2SO4 buffer (pH 7.4) obtained by high-speed centrifugation was loaded onto a Q-Sepharose column (Pharmacia) preequilibrated with the same buffer. The β-lactamase activity was recovered in the flowthrough fraction, dialyzed against 20 mM Tris–H2SO4 buffer (pH 9.4), and loaded onto a Q-Sepharose column equilibrated with the same buffer. The β-lactamase activity was eluted with K2SO4 at a concentration of 50 mM. The β-lactamase peak was dialyzed in 20 mM Tris–H2SO4 buffer (pH 7.4) and concentrated with a Vivaspin 1000 column (Sartorius, Göttingen, Germany). The OXA-32-specific activity against benzylpenicillin was determined as reported previously (17) and was 3,900 mU · mg of protein−1 with a 65-fold increase in purification. OXA-32 had activity against several β-lactams including ceftazidime (Table 2). The kcat/Km value for ceftazidime could not be precisely determined due to an affinity coefficient that was too low (i.e., high Km value) (Table 2).
TABLE 2.
Kinetic parameters for the purified OXA-32 β-lactamasea
| Substrate | kcat | Km (μM) | kcat/Km |
|---|---|---|---|
| Benzylpenicillin | 3.5 | 45 | 80 |
| Cloxacillin | 2.5 | 110 | 20 |
| Amoxicillin | 1.5 | 220 | 70 |
| Ticarcillin | 1 | 60 | 15 |
| Piperacillin | 3 | 155 | 20 |
| Cephalothin | 3 | 60 | 50 |
| Cephaloridine | 2 | 360 | 6 |
| Cefotaxime | —b | — | — |
| Ceftazidime | Hc | >3,000 | H |
| Cefpirome | — | — | — |
| Aztreonam | — | — | — |
Data are the means of three independent experiments. Standard deviations were within 10%.
—, no detectable hydrolysis.
H, hydrolyzed, but kinetic parameters could not be determined due to high Km value.
To further compare the hydrolytic properties of OXA-32 and OXA-2, a site-directed mutagenesis protocol was used as described by the manufacturer (Quick Change Site-Directed mutagenesis kit; Stratagene) to change the Ile to Leu at position 169 of OXA-32, thus restoring the OXA-2 sequence. The nucleotide change in the mutant was checked by sequence analysis. Two isogenic E. coli DH10B strains were obtained containing recombinant plasmids pPG13 and pPG14 expressing OXA-32 and OXA-2, respectively. The MICs of β-lactams for E. coli DH10B(pPG13) compared to those for E. coli DH10B(pPG14) showed that E. coli DH10B(pPG13) had decreased susceptibility to ceftazidime but increased susceptibilities to penicillins and narrow-spectrum cephalosporins (Table 1). The relative activities of β-lactamase extracts of cultures of E. coli DH10B harboring pPG13 and pPG14 were determined with several β-lactams as substrates, as described previously (10). Specific activities for benzylpenicillin were 60 and 2,660 mU · mg of protein−1 for OXA-32 and OXA-2, respectively. The relative specific activities of β-lactams compared to those of benzylpenicillin mirrored the MIC results (Table 3). Thus, the role of the Leu169Ile substitution was established for extension of the resistance profile of OXA-32.
TABLE 3.
Relative activities and 50% inhibitory concentrations determined with β-lactamase extracts of cultures of E. coli DH10B harboring recombinant plasmids pPG13 (OXA-32) and pPG14 (OXA-2)
| Substrate | Relative activitya
|
IC50 (μM)
|
||
|---|---|---|---|---|
| OXA-32 | OXA-2 | OXA-32 | OXA-2 | |
| Benzylpenicillin | 100 | 100 | ||
| Ticarcillin | <1 | 6 | ||
| Piperacillin | 86 | <1 | ||
| Cephalothin | 330 | 35 | ||
| Ceftazidime | 95 | 2 | ||
| Cefotaxime | <1 | <1 | ||
| Cloxacillin | 12 | 6 | ||
| Clavulanate | 8 | 3 | ||
| Tazobactam | 0.05 | 0.05 | ||
| Imipenem | 0.01 | 0.1 | ||
| Cefoxitin | 0.01 | 0.5 | ||
Specific activities were determined relative to those of benzylpenicillin, which were set at 100 for each enzyme. Standard deviations were within 15% (means of three independent determinations).
Inhibition studies, as measured by 50% inhibitory concentrations (IC50s) with benzylpenicillin (100 μM) as the substrate, were performed as described previously (16). OXA-32 and OXA-2 activities were weakly inhibited by clavulanic acid, while tazobactam was an efficient inhibitor, as has been reported for most oxacillinases (Table 3). Imipenem was a better inhibitor of OXA-32 activity than of OXA-2 activity, as reported previously for the OXA-10 derivatives (11). Cefoxitin was, surprisingly, a strong inhibitor of OXA-32. A synergy between cefoxitin and ceftazidime was easily detected by disk diffusion with OXA-32-producing E. coli DH10B (data not shown). To the best of our knowledge, this is the first report on the inhibitory properties of cephamycin against class D enzymes (9).
A second ORF was identified that corresponded to the aacA4 gene located downstream of blaOXA-32. It encoded an aminoglycoside acetyltransferase AAC(6′)-Ib variant named AAC(6′)-Ib9 with a Leu-to-Ser change at position 119 with respect to the amino acid sequence of the other AAC(6′)-Ib protein (10). This amino acid change had been associated with a shift from amikacin to gentamicin resistance in vitro after site-directed mutagenesis (18). AAC(6′)-Ib9 was also identified in several gram-negative clinical isolates including a P. aeruginosa strain that possessed a class 1 integron containing a gene cassette encoding the OXA-10 extended derivative OXA-19 (8, 10). As expected, E. coli DH10B(pPG13) was resistant to gentamicin and susceptible to amikacin according to antibiotic susceptibility testing by disk diffusion (data not shown).
A structure of a class 1 integron surrounded the blaOXA-32 gene with (i) a 5′ coding sequence (5′-CS) containing a class 1 integrase gene, (ii) an attI1 recombination site, and (iii) a 3′-CS containing the qacEΔ1 cassette. The oxa-32 gene cassette had a core site (5′-GTTGGGC-3′), a perfect inverse core site (5′-GCCCAAC-3′), and a 59-be site made up of 70 bp starting inside the 3′-end coding sequence of the gene. It was almost identical to that found for the oxa-2 cassette (substitution of A to T at position 819 of the gene [3]) (data not shown). The 59-be sequence of the aacA4 gene cassette was as described previously (10).
Extraction of plasmid from the P. aeruginosa CY-1 culture with the plasmid DNA MaxiKit (Qiagen, Courtaboeuf, France) gave a plasmid of ca. 250 kb designated pGER-1. Plasmid pGER-1 carried the blaOXA-32 gene, according to hybridization results (Southern blotting technique, as described previously [17, 20]) obtained by PCR with an 828-bp internal probe for blaOXA-32 (primer OXA-A [5′-ATGGCAATCCGAATCTTCGC-3′] and primer OXA-B [5′-TTATCGCGCTGCGTCCGAGT-3′]). Conjugation experiments, performed as described previously (17), showed that pGER-1 was conjugated from P. aeruginosa CY-1 to ciprofloxacin-resistant reference strain P. aeruginosa PU21 obtained in vitro. The MICs of β-lactams for P. aeruginosa PU21(pGER-1) mirrored those for P. aeruginosa CY-1 (Table 1).
Finally, this work reports on the second extended-spectrum derivative of the OXA-2 β-lactamase whose gene was located on a plasmid and an integron.
Nucleotide sequence accession number.
The nucleotide sequences of the oxa-32 gene and the corresponding class 1 integron have been assigned to the EMBL GenBank database under accession number AF315351.
Acknowledgments
This work was funded by the Ministère de l’Education Nationale et de la Recherche (UPRES, grant JE-2227), Université Paris XI, Paris, France.
REFERENCES
- 1.Bou, G., A. Oliver, and J. Martinez-Beltran. 2000. OXA-24, a novel class D beta-lactamase with carbapenemase activity in an Acinetobacter baumannii clinical strain. Antimicrob. Agents Chemother. 44:1556–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Couture, F., J. Lachapelle, and R. C. Lévesque. 1992. Phylogeny of LCR-1 and OXA-5 with class A and class D β-lactamases. Mol. Microbiol. 6:1693–1705. [DOI] [PubMed] [Google Scholar]
- 3.Dale, J. W., D. Godwin, D. Mossakowska, P. Stephenson, and S. Wall. 1985. Sequence of the OXA-2 beta-lactamase: comparison with other penicillin-reactive enzymes. FEBS Lett. 191:39–44 [DOI] [PubMed] [Google Scholar]
- 4.Danel, F., L. M. C. Hall, D. Gür, and D. M. Livermore. 1997. OXA-15, an extended-spectrum variant of OXA-2 β-lactamase, isolated from a Pseudomonas aeruginosa strain. Antimicrob. Agents Chemother. 41:785–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donald, H. M., W. Scaife, S. G. Amyes, and H.-K. Young. 2000. Sequence analysis of ARI-1, a novel OXA β-lactamase responsible for imipenem resistance in Acinetobacter baumannii 6B92. Antimicrob. Agents Chemother. 44:196–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fluit, A. C., and F. J. Schmitz. 1999. Class 1 integrons, gene cassettes, mobility, and epidemiology. Eur. J. Clin. Microbiol. Infect. Dis. 18:761–770. [DOI] [PubMed] [Google Scholar]
- 7.Joris, B., P. Ledent, O. Dideberg, E. Fonzé, J. Lamotte-Brasseur, J. A. Kelly, J. M. Ghuysen, and J.-M. Frère. 1991. Comparison of the sequences of class A β-lactamases and the secondary structure elements of penicillin-recognizing proteins. Antimicrob. Agents Chemother. 35:2294–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lambert, T., M. C. Ploy, and P. Courvalin. 1994. A spontaneous point mutation in the aac(6′)-Ib′ gene results in altered substrate specificity of aminoglycoside 6′-N-acetyltransferase of a Pseudomonas fluorescens strain. FEMS Microbiol. Lett. 115:297–304. [DOI] [PubMed] [Google Scholar]
- 9.Ledent, P., X. Raquet, B. Joris, J. Van Beeumen, and J.-M. Frère. 1993. A comparative study of class-D β-lactamases. Biochem. J. 292:555–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mugnier, P., I. Casin, A. T. Bouthors, and E. Collatz. 1998. Novel OXA-10-derived extended-spectrum β-lactamases selected in vivo or in vitro. Antimicrob. Agents Chemother. 42:3113–3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mugnier, P., I. Podglajen, F. W. Goldstein, and E. Collatz. 1998. Carbapenem as inhibitors of OXA-13, a novel integron-encoded β-lactamase in Pseudomonas aeruginosa. Microbiology 144:1021–1031. [DOI] [PubMed] [Google Scholar]
- 12.Naas, T., and P. Nordmann. 1999. OXA-type β-lactamases. Curr. Pharm. Design 5:865–879. [PubMed] [Google Scholar]
- 13.Nordmann, P., and M. Guibert. 1998. Extended-spectrum β-lactamases in Pseudomonas aeruginosa. J. Antimicrob. Chemother. 42:128–131. [DOI] [PubMed] [Google Scholar]
- 14.Ouellette, M., L. Bissonnette, and P. H. Roy. 1987. Precise insertion of antibiotic resistance determinants into Tn21-like transposons: nucleotide sequence of the OXA-1 β-lactamase gene. Proc. Natl. Acad. Sci. USA 84:7378–7382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Philippon, A. M., G. C. Paul, and G. A. Jacoby. 1986. New plasmid-mediated oxacillin-hydrolyzing β-lactamase in Pseudomonas aeruginosa. J. Antimicrob. Chemother. 17:415–422. [DOI] [PubMed] [Google Scholar]
- 16.Philippon, L. N., T. Naas, A.-T. Bouthors, V. Barakett, and P. Nordmann. 1997. OXA-18, a class D clavulanic acid-inhibited extended-spectrum β-lactamase from Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 41:2188–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poirel, L., D. Girlich, T. Naas, and P. Nordmann. 2001. OXA-28, an extended-spectrum variant of OXA-10 β-lactamase from Pseudomonas aeruginosa and its plasmid- and integron-located gene. Antimicrob. Agents Chemother. 45:447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rather, P. N., H. Munayyer, P. A. Mann, R. S. Hare, G. H. Miller, and K. J. Shaw. 1992. Genetic analysis of bacterial acetyltransferase: identification of amino acids determining the specificities of the aminoglycoside 6′-N-acetyltransferase Ib and IIa proteins. J. Bacteriol. 174:3196–3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Recchia, G. D., and R. M. Hall. 1995. Gene cassettes: a new class of mobile elements. Microbiology 141:3015–3027. [DOI] [PubMed] [Google Scholar]
- 20.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.