Abstract
The immediate efficacies of two oral dosage regimens of artemisinin were investigated in 77 male and female adult Vietnamese falciparum malaria patients randomly assigned to treatment with either 500 mg of artemisinin daily for 5 days (group A; n = 40) or artemisinin at a dose of 100 mg per day for 2 days, with the dose increased to 250 mg per day for 2 consecutive days and with a final dose of 500 mg on the fifth day (group B; n = 37). Parasitemia was monitored every 4 h. The average parasite clearance time was longer in group B than in group A (means ± standard deviations, 50 ± 23 and 34 ± 14 h, respectively; P < 0.01). Artemisinin concentrations in saliva samples obtained on days 1 and 5 were quantified by high-performance liquid chromatography. The average oral clearance, based on saliva drug concentrations in group B patients, was twofold higher than that in group A patients on day 1 (P < 0.01), with no differences in drug half-lives (P = 0.40), indicating a saturable first-pass metabolism. Female patients had higher oral clearance values on day 1. Artemisinin's pharmacokinetic parameters were similar on day 5 in both groups, although a significant increase in oral clearance from day 1 to day 5 was evident. Thus, artemisinin exhibited both dose- and time-dependent pharmacokinetics. The escalating dose studied did not result in higher artemisinin concentrations toward the end of the treatment period.
Artemisinin and its semisynthetic family of derivatives are the most potent antimalarials available for treatment of falciparum malaria infections (15, 25, 26). Artemisinin has a short elimination half-life, a rapid onset of action, no major side effects, and markedly induces of its own metabolic elimination, resulting in five- to sevenfold decreases in its concentrations over 5 to 7 days of administration in both malaria patients and healthy subjects (1, 3-5). A major drawback with artemisinin and its derivatives is the high recrudescence rates within 2 to 3 weeks after monotherapy (14, 17). It has proved difficult in field studies to determine whether the relapsed malaria episodes are due to new infections or the persistence of the previous one. However, true recrudescence has been described in patients kept in vector-free environments for longer periods of time (12, 13, 16). There is also evidence of true recrudescence, as determined by a PCR technique with patients treated with a combination of artemether and benflumetol (10) as well as artesunate alone (18). It has been suggested that the occurrence of recrudescence may partly be due to the decreasing artemisinin concentrations toward the end of treatment (4). Thus, the time-dependent kinetics of the drug can be of importance in dosing suggestions.
Reports concerning dose-response studies with the artemisinin endoperoxides are lacking. Rectal administration of artemisinin was shown to have efficacy comparable to that of oral treatment in terms of the parasite clearance time (PCT) and the fever subsidence time (FST) in patients with uncomplicated falciparum malaria (4). This was despite the observation that artemisinin areas under the concentration-time curves (AUCs) after rectal administration were in general only 30% of those after oral intake. This raises doubts about how justified the standard dosage regimens have been and whether a 500-mg daily dose may be unnecessarily high to achieve the immediate effects of the drug.
Saliva artemisinin concentrations are highly correlated with the venous and capillary plasma artemisinin concentrations, making saliva a body fluid feasible for use in pharmacokinetic (PK) investigations of the compound (7, 21).
The aim of the present study was to investigate whether a dosing regimen consisting of low initial doses and then escalating doses of artemisinin was as efficacious as the standard high-dose regimen with respect to the immediate clinical end points. It was also of interest to evaluate if lower initial doses of artemisinin lead to a less pronounced induction of its elimination in patients with uncomplicated malaria. If this is the case, this would result in higher drug concentrations toward the end of the treatment period, which in turn could hypothetically reduce recrudescence rates.
MATERIALS AND METHODS
Study design.
Seventy-seven male and female adults with uncomplicated falciparum malaria were included in this double-blind, comparative, parallel-group study. Block randomization was applied for treatment arm allocation. The study took place at the health care facilities of the Phú Rieng Rubber Plantation in Binh Phuoc Province, Vietnam, which is 130 km north of Ho Chi Minh City, Vietnam. Written, informed consent was obtained from each patient prior to inclusion in the study. Permission for the study was obtained from the Ministry of Health, Hanoi, Vietnam; the Ethics Committee of the Medical Faculty of Uppsala University, Uppsala, Sweden; and the Medical Products Agency, Uppsala, Sweden.
Each patient had a peripheral asexual parasite density of more than 1,000 parasites/μl of blood and a sublingual body temperature above 37.5°C upon admission. Parasite counts were determined on Giemsa-stained thick blood films and were recorded as the number of parasites per 300 white blood cells. The following were exclusion criteria: current medication with other drugs; evidence of a cardiovascular, endocrine, gastrointestinal, hematological, hepatic, renal, or respiratory disorder; a hemoglobin level below 80 g/liter; symptoms of cerebral malaria; and antimalarial treatment 3 weeks prior to study inclusion, according to the patients when they were asked. Moreover, patients unable to communicate well with the domestic investigators were excluded from the study.
Artemisinin was purchased from the Institute of Materia Medica (Hanoi, Vietnam). Three different doses (50, 125, and 250 mg) were prepared in hard gelatin capsules, with cellulose used as bulk material and with all doses having identical appearances (The National Corporation of Swedish Pharmacies [Apoteket AB], Stockholm, Sweden). All patients received the same total number of capsules on each occasion. All capsules were administered under the supervision of study team members, with ingestions followed by the intake of 100 ml of water and rinsing of the mouth with another 100 ml of water. Solid food was avoided 2 h before and 2 h after drug intake on the 2 sampling days.
Patients enrolled in group A (standard-dose group; n = 40) received the standard dose of artemisinin, while those enrolled in group B (escalating-dose group; n = 37) were treated according to an escalating-dose scheme (Table 1). On the first and the last days the doses were administered as single doses to both groups to ensure detectable saliva drug levels, while on the second, third, and fourth days the doses were administered in the morning and evening, as generally implemented in clinical settings at the time of the study. All patients were hospitalized for the duration of drug treatment. Patients were asked to return to the hospital 3 weeks after the start of treatment for a parasite test.
TABLE 1.
Artemisinin doses in the two treatment arms
| Dosing regimen | Dosea
|
||||
|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | |
| Standard | 2 × 250 | 250 × 2 | 250 × 2 | 250 × 2 | 2 × 250 |
| Escalating | 2 × 50 | 50 × 2 | 125 × 2 | 125 × 2 | 2 × 250 |
On days 1 and 5 the drug was given as a single dose (in milligrams) of two capsules. On the other days the dose (in milligrams) was divided and given on two occasions.
The target number of patients included in each group (n = 36) was calculated to enable the detection of an 8-h difference in the PCT between the two treatment groups at a significance level of 0.05 and with a power of 0.80. Each patient not completing the 5-day treatment course was replaced by two new patients, who were randomly allocated to each study arm in order to maintain the blindness of the study.
Clinical assessments.
The parasite count in finger capillary blood was determined immediately before and every 4 h after the start of treatment until three consecutive smears were negative. The time until the first (of three) negative smear was defined as the PCT. Body temperature was simultaneously recorded sublingually until it was normal. FST was defined as the time from drug intake until the time that the body temperature fell below 37°C.
Saliva sampling.
Two milliliters of unstimulated saliva was collected from each patient and placed in 3.6-ml plastic cryotubes (Nunc, Hereford, England) at 5 min before and at 30, 60, 90, 120, 150, 180, 240, 300, 360, 420, and 480 min after drug administration on days 1 and 5.
All samples were immediately frozen and kept at −20°C prior to transport to Sweden, where they were transferred within 2 months to −80°C until artemisinin quantification.
Bioanalytical assay.
Each patient's samples were analyzed on the same occasion. Saliva artemisinin concentrations were quantified by high-performance liquid chromatography-UV detection with a column-switching system that allowed the direct injection of samples (8). The lowest concentrations quantified by the system were 2 ng/ml (intraday repeatability coefficient of variation, 11%; injection volume, 1.0 ml).
Data evaluation.
The maximum saliva artemisinin concentration (Cmax) and the time to reach that concentration (Tmax) were taken directly from the observed concentration-time data for each individual. To adjust for the differences in the given doses between the two groups on day 1 (100 and 500 mg in the escalating- and standard-dosing groups, respectively) during the statistical analysis, the Cmax values for patients in the escalating-dose group were multiplied by 5 on the first treatment day (dose-adjusted Cmax). The artemisinin elimination rate constant (k) was estimated for each individual by log-linear, ordinary-least-squares regression of three to five terminal saliva concentration-time datum points. The terminal half-life (t1/2) was calculated as ln2/k. The saliva AUC from the time of drug administration to the time of the last sample with quantifiable artemisinin concentrations (AUC0-t) was calculated by the linear trapezoidal method for the ascending phase of the curve and by use of the log-linear trapezoidal rule for the descending phase of the curve. The AUC from the last time of quantification of parasite in a sample to infinity (AUCt-∞) was estimated by dividing the last predicted concentration by k. The AUC from time zero to infinity (AUC0-∞) was calculated as the sum of AUC0-t and AUCt-∞. The oral clearance based on the concentrations in saliva (CLs/F) for each subject was derived as the dose divided by AUC0-∞. The mean residence time (MRT) for each patient was calculated as the AUMC/AUC0-∞, where AUMC is the area under the first moment-versus-time curve and was calculated by the linear trapezoidal rule with the residual area from the last sampling time point extrapolated by Ccalc/k2, where Ccalc is the calculated concentration.
PCT50 was defined as the time required for the largest number of parasites observed to decrease by 50%. Parasite mean residence time (PMRT), an estimate of the average time that a parasite remains in the body, was calculated as described previously (4). Parasite AUCs (PAUCs) were calculated by using the number of remaining parasite-versus-time curves, as described above for saliva AUCs.
Statistical evaluation.
All statistical tests were applied by using StatView (version 5.0; SAS Institute Inc., Cary, N.C.), unless stated otherwise. Multivariate analysis of covariance (MANCOVA) with Fisher's partial least-significant-difference post hoc test was used for evaluation of the differences in continuous variables between the two groups (24). The sex distribution was investigated by the chi-square test. The data set was divided into demographic, PK, and pharmacodynamic (PD) variables. The demographic data consisted of age, body weight, initial number of parasites, and sex. In the PK analysis differences between the two groups with respect to CLs/F, t1/2, dose-adjusted Cmax, Tmax, and MRT on the first and the last treatment days were investigated. Differences between the two groups in the relative change in CLs/F from saliva between the first and the last study days (intraindividual oral CLs, day 1/oral CLs, day 5 ratios) were tested. Body weight and sex were tested as covariates in all PK analyses. The variables used in the PD analyses included PCT, PCT50, PMRT, PAUC, and FST. Age and the logarithm of the largest number of parasites observed were tested as covariates. The level of significance was set at 0.05 (two tailed) for all the comparisons described above.
Multiple t tests with sequentially rejective Bonferroni adjustment of the P values (9; T. Gordi and H. J. Khamis, submitted for publication) were applied to test the differences in CLs/F, t1/2, dose-adjusted Cmax, Tmax, and MRT on days 1 and 5 within each group. The overall level of significance was set equal to 0.05 (two tailed).
A canonical correlation analysis (24) was performed (SAS, version 6.12; SAS Institute Inc.) to analyze the relationship between PK (CLs/Fday 1, Cmax, day 1, MRTday 1) and PD (PCT, PCT50, PMRT, PAUC, and FST) variables. Visual inspections of different scattergrams were also performed to search for relationships other than linear relationships between the variables.
RESULTS
Demographics.
No significant differences between the two treatment groups with respect to the initial number of parasites, body weight, age, and sex distribution were in evidence (Table 2). Artemisinin was well tolerated by all patients. Data for two patients in the escalating-dose group were excluded from the data analysis due to either uncompleted treatment or dosing for a shorter interval than that permitted in the study protocol, respectively. These patients were replaced by four new patients, randomly allocated in pairs to either dosing regimen.
TABLE 2.
Demographic and clinical response parameters for Vietnamese adults with uncomplicated falciparum malaria orally treated with artemisinin by a standard- or escalating-dose regimen
| Parameter | Standard dosing | Escalating-dose dosing | P value |
|---|---|---|---|
| Demographics | |||
| Gender (no. of males, no. of females) | 28, 12 | 25, 12 | >0.99 |
| Age (yr)a | 27.7 ± 10.2 | 28.1 ± 10.5 | 0.90 |
| Body wt (kg)a | 49.8 ± 6.1 | 48.8 ± 6.2 | 0.90 |
| Initial parasitemia (no. of parasites/μl of blood) | |||
| Geometric mean | 8,290 | 5,408 | 0.90 |
| Range | 1,120-137,920 | 1,012-208,320 | |
| Clinical response | |||
| PCT50 (h)b | 10.8 ± 3.3 (9.7, 11.9) | 11.2 ± 5.2 (9.5, 13.9) | 0.85 |
| PCT (h)b | 34.1 ± 14.1 (29.6, 38.6) | 50.2c ± 23.4 (42.3, 58.0) | <0.01 |
| PMRT (h)b | 7.3 ± 2.7 (6.5, 8.2) | 11.1 ± 7.0 (8.8, 13.4) | <0.01 |
| PAUC (no. of parasites · h/μl of blood)d | 271,522 ± 353,430 (138,971) | 536,359c ± 1,724,730 (100,386) | 0.09 |
| FST (h)b | 24.8 ± 9.6 (21.7, 27.9) | 25.7 ± 15.9 (20.4, 31.0) | 0.60 |
Values are means ± standard deviations.
Values are means ± standard deviations (95% confidence intervals).
Including the arbitrary value of 120 h for one patient.
Values are means ± standard deviations (geometric means).
Efficacy.
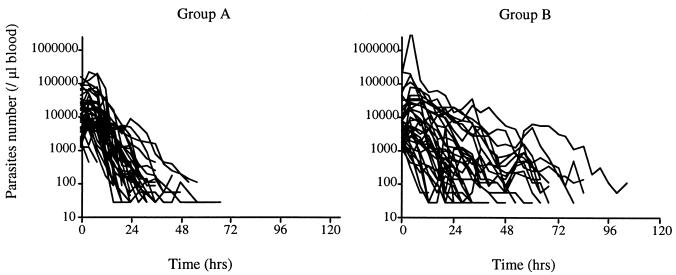
The patients in the standard-dose group had significantly shorter mean PCTs and PMRTs compared to those for the patients in the escalating-dose group (P < 0.01) (Fig. 1; Table 2). No statistically significant differences in FST, PCT50, or PAUC were found between the two groups. One patient in the escalating-dose group did not clear the parasites up to 104 h after intake of the first dose. In the statistical tests, the PCT for this patient was given an arbitrary value of 120 h. In a sensitivity analysis, the outcomes of the tests did not differ with or without the inclusion of the data for this patient.
FIG. 1.
Number of asexual P. falciparum parasites per microliter of blood in adult Vietnamese patients treated with either a standard oral regimen of 500 mg of artemisinin for 5 days (group A) or an escalating-dosage oral regimen of 100 mg on days 1 and 2, 250 mg on days 3 and 4, and a final dose of 500 mg on day 5 (group B).
The logarithm of the highest level of parasitemia had a significant effect on PCT and PAUC when it was used as covariate in the MANCOVA (P < 0.01). It also exhibited an influence on FST, with a P value close to the level of significance (P = 0.07), whereas PCT50 and PMRT were unaffected (P = 0.76, and 0.74, respectively). No significant effect of age was found in any of the variables used in the PD analysis (PCT, PCT50, PMRT, PAUC, and FST).
A total of 31 patients returned for parasite control within 3 weeks after treatment termination. Among these 31 patients, Plasmodium falciparum parasites were detected in 6 of 17 patients in the standard-dose group and 7 of 14 patients in the escalating-dose group. No other plasmodium types were detected in any of the returning patients. All recurring infections were treated with mefloquine (Lariam; Roche, Basel, Switzerland).
Pharmacokinetics.
Due to low saliva artemisinin concentrations, pharmacokinetic parameters were based on data for 43 male patients (24 and 19 patients in the standard- and escalating-dose groups, respectively) and 16 female patients (7 and 9 in the standard- and escalating-dose groups, respectively). Artemisinin concentrations in samples taken 30 min after drug intake were abruptly high in several patients. In the final analysis, all concentration data from this time point were considered unreliable and were not used in the data evaluation.
A MANCOVA with CLs/F, t1/2, dose-adjusted Cmax, Tmax, and MRT as the dependent variables and dosing group as the independent variable indicated significant differences between the two groups on the first treatment day (P < 0.01). This overall P value was due to differences in CLs/F values between the two groups (P < 0.01), with higher values obtained for those in the escalating-dose group. A P value close to the level of significance was obtained for MRT (P = 0.05), with larger values observed for patients in the standard-dose group (Table 3). No significant differences were found with respect to other variables. Both sex and body weight showed significant influences on the difference in CLs/F between the groups (P < 0.01 for both sex and body weight), with lower values for female patients and higher values with increased weight. P values close to the level of significance were found for the effect of the two covariates on the dose-adjusted Cmaxs (P = 0.07 for both sex and body weight). There were no significant differences in PK parameters between the two groups on day 5 (P = 0.96). However, a significant difference in the relative changes in the CLs/F values (CLs/Fday 1 divided by CLs/Fday 5) was observed between the two groups (P < 0.01), with no effect by sex (P = 0.17) or body weight (P = 0.84).
TABLE 3.
Pharmacokinetic parameters for Vietnamese male and female adults with uncomplicated falciparum malaria orally treated with artemisinin by a standard- or escalating-dose regimena
| Dosing regimen (dose [mg]) | CLs/F (liter/h)
|
P value for CLs/Fb |
t1/2 h
|
P value for t1/2b |
Cmax/Dc
|
P value for Cmax/Db |
Tmax (h)
|
P value for Tmaxb | MRT (h)
|
P value for MRTb | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | 95% CId | Mean ± SD | 95% CI | Mean ± SD | 95% CI | Mean ± SD | 95% CI | Mean ± SD | 95% CI | ||||||
| Standard | <0.01 | 0.99 | <0.01 | 0.14 | 0.98 | ||||||||||
| Day 1 (500) | 1,019 ± 633e | 783, 1,255 | 1.3 ± 1.1e | 0.9, 1.7 | 244 ± 202e | 162, 311 | 2.9 ± 1.9e | 2.3, 3.8 | 4.2 ± 1.4e | 3.7, 4.8 | |||||
| Day 5 (500) | 8,104 ± 7,837f | 5,971, 12,895 | 1.4 ± 2.4f | 0.8, 1.7 | 53 ± 47f | 35, 71 | 2.6 ± 1.2f | 2.1, 3.0 | 4.4 ± 3.9f | 2.8, 5.8 | |||||
| Escalating | <0.01 | 0.87 | <0.01 | 0.19 | 0.48 | ||||||||||
| Day 1 (100) | 2,019 ± 1,277f | 1,503, 2,535 | 1.1 ± 0.7f | 0.8, 1.4 | 185 ± 194e | 93, 232 | 2.8 ± 1.5f | 2.4, 3.7 | 3.5 ± 1.1f | 3.2, 4.0 | |||||
| Day 5 (500) | 7,160 ± 5,614f | 4,983, 9,336 | 1.1 ± 0.8e | 0.8, 1.4 | 57 ± 43f | 37, 70 | 2.5 ± 1.0f | 2.1, 2.9 | 3.7 ± 1.3e | 3.3, 4.2 | |||||
| P values for D1, D5g | <0.01, 0.79 | 0.40, 0.79 | 0.36, 0.79 | ||||||||||||
The overall level of significance was set at 0.05 (two tailed) in all analyses.
P values for within-group differences on the first and last treatment days.
Cmax/D, dose-adjusted observed maximal concentration.
95% CI, 95% confidence interval.
n = 29 to 31.
n = 26 to 28.
D1 and D5, between-group differences on the first and last treatment days, respectively.
The extrapolated fractions of AUC0-∞ were, on average, 10 and 15% for the standard- and escalating-dose groups, respectively, on day 1 and 12% for both groups on day 5.
Analysis by canonical correlation indicated no evidence for any correlation between the PD variables (PCT, PCT50, PMRT, PAUC, and FST) and any of the PK variables (CLs/Fday 1, Cmax, day 1, MRTday 1), with a P value equal to 0.21. For the variable Cmax, day 1, two outliers were removed from the data set because they were out of the range of the calibration curve. Additionally, there were three outliers in the data set, and these were removed due to the abnormal distribution that they produced. These consisted of one PMRT datum point, one PAUC datum point, and one MRT datum point. Inclusion or exclusion of these datum points did not alter the overall conclusions (P = 0.35 and 0.21, respectively). No trends for any correlation could be detected upon visual inspection of scattergrams of PD versus PK variables.
DISCUSSION
With improvements in analytical systems for the detection of artemisinin and its derivatives in recent years, increased numbers of reports on the PK properties of this family of antimalarials have been published (25). However, the available information has not been used in any dose-finding studies for any of these compounds.
In the present study, the PCT following the use of an escalating-dose regimen was compared with that following the use of standard artemisinin therapy. Moreover, the PKs of artemisinin were studied with saliva samples, with the hypothesis being that lower initial doses of artemisinin would result in less pronounced autoinduction of drug metabolism. The escalating-dose regimen was chosen to somewhat counter the unusual time-dependent PKs of artemisinin. The starting dose chosen for the escalating-dose regimen was based on earlier findings that suggested that lower artemisinin concentrations suffice for the clearance of the parasites (4).
PCT was prolonged in the escalating-dose group by a mean of 16 h compared to that in the group receiving the standard treatment. This is explained by the inadequate artemisinin concentrations in the former group, as manifested by 10-fold lower saliva AUCs and 7-fold lower salivary Cmax values. In general, the prolongation of PCT is undesirable, resulting in an increased level of gametocytogenesis and an increased level of parasite transmission (6, 19).
The number of recurring infections appeared to be similar in the two treatment arms. However, this study was not dimensioned to detect any such difference. One male patient in the escalating-dose group had not cleared the parasites until the last treatment day, although he was symptom-free. The final parasite count in his blood smears was done after his release from the study, and we were unable to locate him after we found parasites in his blood smears. This patient, however, returned to the hospital 10 days after the end of the treatment presenting with renewed symptoms. Falciparum malaria was detected in his blood, and he was successfully treated with mefloquine.
On average, CLs/F was significantly higher in the patients in the escalating-dose group than in the standard-dose group on the first treatment day, with no evidence for any difference in t1/2. The difference observed here might be an indication of a saturable first-pass metabolism, such that the higher dose would have a higher oral bioavailability. The presumably lower bioavailability on day 1 in patients receiving the 100-mg dose resulted in concentrations lower than expected, which probably contributed to the prolonged PCT in this group. With artemisinin exhibiting the characteristics of a highly extracted drug (5), there would be no saturation of the systemic clearance and therefore no differences in t1/2s. Indications of saturable artemisinin kinetics after oral administration have previously been found in healthy subjects (2).
Artemisinin CLs/F values on day 5 did not differ between the two groups. However, these values were significantly higher compared to those on day 1 in both groups (Table 3). The increase is in accordance with earlier findings based on concentrations in plasma in both malaria patients and healthy volunteers (1, 3-5). The extent of the increase in artemisinin CLs/F values has been reported to be six- to sevenfold after standard treatment (1, 4, 22). In our study, the average increase in CLs/F from day 1 to 5 in the patients in the standard-dose group was 5-fold, whereas the average increase in the escalating-dose group was 2.5-fold. However, both groups seem to have reached the maximum induction level, resulting in similar CLs/F values on day 5. MANCOVA detected a significant difference between the groups with respect to the increases in these values. However, the seemingly smaller increase in the patients allocated to the escalating-dose regimen is probably due to the higher CLs/F values on day 1 for this group.
In a population kinetic study, lower CL/F values and volumes of distribution for artemisinin were found in female children (22). However, due to the small number of male and female children involved, sex was not concluded to influence the pediatric artemisinin kinetics. Our results suggest a significant effect of sex on the kinetics of the drug on the first treatment day, with higher CLs/F values for female patients. Artemisinin metabolism in human liver microsomes is mediated primarily by CYP2B6, with a probable secondary contribution of CYP3A4 in individuals with low levels of CYP2B6 expression (23). Reports on differences in the activities of these enzymes by sex are scarce, although indications of higher levels of activity in female subjects have been reported for CYP2A6 (11, 20). Another explanation for the observed effect of sex could be the imbalance in the number of male and female patients. Although MANCOVA analysis is, in general, robust enough to handle such an inequality, a possible statistical misinterpretation could not be ruled out. No effect of sex on CLs/F was in evidence on the last treatment day.
An alternative means for the achievement of higher artemisinin concentrations at the end of a treatment period would be to start with the standard dose of 500 mg and then increase the dose toward the end of the treatment. However, countering of the time-dependent PKs of artemisinin would require doses as high as 2 to 3 g toward the end of the treatment period. No data on the abilities of patients to tolerate artemisinin or its metabolites at such high doses are available, however.
Combination therapies for the treatment of malaria with an artemisinin analogue and other antimalarials with longer t1/2 values have been proposed (17, 26). Such combinations are believed to reduce the risk for the development of resistance. Our findings support previous suggestions on the use of combination therapy because of the complex PK of artemisinin, which are both dose and time dependent. However, artemisinin is probably the least suitable of its class for use in combination chemotherapy due to the potential for drug-drug interactions caused by the induction of drug-metabolizing enzymes.
This is the first reported study on the PKs of artemisinin based solely on saliva samples. The agreement of the results of the study with those presented in previous publications on the nonlinearity of the kinetics of the compound and its autoinduction capacity encourages the use of saliva as a feasible sampling matrix. The utility of this approach lies primarily with the easier sampling procedure compared to that for blood sampling and patients' more ready acceptance of saliva sampling. However, care must be taken in rinsing the mouth properly in order to avoid contamination of the early samples with residual artemisinins, which was the reason that the samples obtained at 30 min were discarded from the PK analysis. Moreover, due to low concentrations in saliva, no artemisinin could be detected in samples from several patients. Analytical systems more sensitive than the present one are a necessity for further investigations based on saliva sampling.
In conclusion, the kinetics of artemisinin were found to exhibit both time and dose dependencies. The escalating-dose regimen with lower initial doses of artemisinin did not result in higher levels of exposure to the drug toward the end of the treatment. This dose regimen resulted in prolonged PCTs, and its clinical implementation is not recommended.
Acknowledgments
The kind assistance of B. Thu, director of the Phú Rieng Rubber Plantation Hospital, is gratefully acknowledged. We also thank H. Khamis for advice on the statistical analyses.
This work was supported in part by the Swedish International Development Cooperation Agency.
REFERENCES
- 1.Alin, M. H., M. Ashton, C. M. Kihamia, G. J. Mtey, and A. Bjorkman. 1996. Multiple dose pharmacokinetics of oral artemisinin and comparison of its efficacy with that of oral artesunate in falciparum malaria patients. Trans. R. Soc. Trop. Med. Hyg. 90:61-65. [DOI] [PubMed] [Google Scholar]
- 2.Ashton, M., T. Gordi, T. N. Hai, N. V. Huong, N. D. Sy, N. T. Nieu, D. X. Huong, M. Johansson, and D. C. Le. 1998. Artemisinin pharmacokinetics in healthy adults after 250, 500 and 1000 mg single oral doses. Biopharm. Drug Dispos. 19:245-250. [DOI] [PubMed] [Google Scholar]
- 3.Ashton, M., T. N. Hai, N. D. Sy, D. X. Huong, N. V. Huong, N. T. Nieu, and L. D. Cong. 1998. Artemisinin pharmacokinetics is time-dependent during repeated oral administration in healthy male adults. Drug Metab. Dispos. 26:25-27. [PubMed] [Google Scholar]
- 4.Ashton, M., N. D. Sy, N. V. Huong, T. Gordi, T. N. Hai, D. X. Huong, N. T. Nieu, and L. D. Cong. 1998. Artemisinin kinetics and dynamics during oral and rectal treatment of uncomplicated malaria. Clin. Pharmacol. Ther. 63:482-493. [DOI] [PubMed] [Google Scholar]
- 5.Ashton, M., N. D. Sy, N. V. Huong, T. Gordi, T. N. Hai, D. C. Thach, M. H. Farah, M. Johansson, and L. D. Cong. 1996. Evidence for time-dependent artemisinin kinetics in adults with uncomplicated malaria. Pharm. Pharmacol. Lett. 6:127-130. [Google Scholar]
- 6.Buckling, A., L. C. Ranford-Cartwright, A. Miles, and A. F. Read. 1999. Chloroquine increases Plasmodium falciparum gametocytogenesis in vitro. Parasitology 118:339-346. [DOI] [PubMed] [Google Scholar]
- 7.Gordi, T., T. N. Hai, N. M. Hoai, M. Thyberg, and M. Ashton. 2000. Use of saliva and capillary blood samples as substitutes for venous blood sampling in pharmacokinetic investigations of artemisinin. Eur J. Clin. Pharmacol 56:561-566. [DOI] [PubMed] [Google Scholar]
- 8.Gordi, T., E. Nielsen, Z. Yu, D. Westerlund, and M. Ashton. 2000. Direct analysis of artemisinin in plasma and saliva using coupled- column high-performance liquid chromatography with a restricted-access material pre-column. J. Chromatogr. B Biomed. Sci. Appl. 742:155-162. [DOI] [PubMed] [Google Scholar]
- 9.Holm, S. 1979. A simple sequentially rejective multiple test procedure. Scand. J. Statist. 6:65-70. [Google Scholar]
- 10.Irion, A., I. Felger, S. Abdulla, T. Smith, R. Mull, M. Tanner, C. Hatz, and H. P. Beck. 1998. Distinction of recrudescences from new infections by PCR-RFLP analysis in a comparative trial of CGP 56 697 and chloroquine in Tanzanian children. Trop. Med. Int. Health 3:490-497. [DOI] [PubMed] [Google Scholar]
- 11.Iscan, M., H. Rostami, T. Guray, O. Pelkonen, and A. Rautio. 1994. Interindividual variability of coumarin 7-hydroxylation in a Turkish population. Eur. J. Clin. Pharmacol. 47:315-318. [DOI] [PubMed] [Google Scholar]
- 12.Karbwang, J., K. N. Bangchang, A. Thanavibul, D. Bunnag, T. Chongsuphajaisiddhi, and T. Harinasuta. 1992. Comparison of oral artemether and mefloquine in acute uncomplicated falciparum malaria. Lancet 340:1245-1248. [DOI] [PubMed] [Google Scholar]
- 13.Karbwang, J., K. Na-Bangchang, A. Thanavibul, and P. Molunto. 1998. Plasma concentrations of artemether and its major plasma metabolite, dihydroartemisinin, following a 5-day regimen of oral artemether, in patients with uncomplicated falciparum malaria. Ann. Trop. Med. Parasitol. 92:31-36. [DOI] [PubMed] [Google Scholar]
- 14.Li, G. Q. 1989. Clinical studies on artemisinin suppository and on artesunate and artemether, p. 69-73. In S. Jiaxiang (ed.), Antimalarial drug development in China. National Institute of Pharmaceutical Research and Development, Beijing, People's Republic of China.
- 15.Meshnick, S. R., T. E. Taylor, and S. Kamchonwongpaisan. 1996. Artemisinin and the antimalarial endoperoxides: from herbal remedy to targeted chemotherapy. Microbiol. Rev. 60:301-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Na-Bangchang, K., J. Karbwang, P. A. Palacios, R. Ubalee, S. Saengtertsilapachai, and W. H. Wernsdorfer. 2000. Pharmacokinetics and bioequivalence evaluation of three commercial tablet formulations of mefloquine when given in combination with dihydroartemisinin in patients with acute uncomplicated falciparum malaria. Eur. J. Clin. Pharmacol. 55:743-748. [DOI] [PubMed] [Google Scholar]
- 17.Nosten, F., T. T. Hien, and N. J. White. 1998. Use of artemisinin derivatives for the control of malaria. Med. Trop. (Mars) 58:45-49. [PubMed] [Google Scholar]
- 18.Price, R., M. van Vugt, F. Nosten, C. Luxemburger, A. Brockman, L. Phaipun, T. Chongsuphajaisiddhi, and N. White. 1998. Artesunate versus artemether for the treatment of recrudescent multidrug-resistant falciparum malaria. Am. J. Trop. Med. Hyg. 59:883-888. [DOI] [PubMed] [Google Scholar]
- 19.Price, R. N., F. Nosten, C. Luxemburger, F. O. ter Kuile, L. Paiphun, T. Chongsuphajaisiddhi, and N. J. White. 1996. Effects of artemisinin derivatives on malaria transmissibility. Lancet 347:1654-1658. [DOI] [PubMed] [Google Scholar]
- 20.Rautio, A., H. Kraul, A. Kojo, E. Salmela, and O. Pelkonen. 1992. Interindividual variability of coumarin 7-hydroxylation in healthy volunteers. Pharmacogenetics 2:227-233. [DOI] [PubMed] [Google Scholar]
- 21.Sidhu, J. S., and M. Ashton. 1997. Single-dose, comparative study of venous, capillary and salivary artemisinin concentrations in healthy, male adults. Am. J. Trop. Med. Hyg. 56:13-16. [DOI] [PubMed] [Google Scholar]
- 22.Sidhu, J. S., M. Ashton, N. V. Huong, T. N. Hai, M. O. Karlsson, N. D. Sy, E. N. Jonsson, and L. D. Cong. 1998. Artemisinin population pharmacokinetics in children and adults with uncomplicated falciparum malaria. Br. J. Clin. Pharmacol. 45:347-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Svensson, U. S., M. Ashton, N. H. Trinh, L. Bertilsson, X. H. Dinh, V. H. Nguyen, T. N. Nguyen, D. S. Nguyen, J. Lykkesfeldt, and D. C. Le. 1998. Artemisinin induces omeprazole metabolism in human beings. Clin. Pharmacol. Ther. 64:160-167. [DOI] [PubMed] [Google Scholar]
- 24.Tabachnick, B. G., and L. S. Fidell. 1996. Using multivariate statistics, 3rd ed. HarperCollins College Publishers, New York, N.Y.
- 25.van Agtmael, M. A., T. A. Eggelte, and C. J. van Boxtel. 1999. Artemisinin drugs in the treatment of malaria: from medicinal herb to registered medication. Trends Pharmacol. Sci. 20:199-205. [DOI] [PubMed] [Google Scholar]
- 26.White, N. 1999. Antimalarial drug resistance and combination chemotherapy. Philos. Trans. R. Soc. Lond. B Biol. Sci. 354:739-749. [DOI] [PMC free article] [PubMed] [Google Scholar]