Abstract
The present study tracks the development of low-level azole resistance in in vitro fluconazole-adapted strains of Candida albicans, which were obtained by serially passaging a fluconazole-susceptible dose-dependent strain, YO1-16 (fluconazole MIC, 16 μg ml−1) in increasing concentrations of fluconazole, resulting in strains YO1-32 (fluconazole MIC, 32 μg ml−1) and YO1-64 (MIC, 64 μg ml−1). We show that acquired resistance to fluconazole in this series of isolates is not a random process but is a gradually evolved complex phenomenon that involves multiple changes, which included the overexpression of ABC transporter genes, e.g., CDR1 and CDR2, and the azole target enzyme, ERG11. The sequential rise in fluconazole MICs in these isolates was also accompanied by cross-resistance to other azoles and terbinafine. Interestingly, fluorescent polarization measurements performed by using the fluorescent probe 1,6-diphenyl-1,3,5-hexatriene revealed that there was a gradual increase in membrane fluidity of adapted strains. The increase in fluidity was reflected by observed change in membrane order, which was considerably decreased (decrease in fluorescence polarization values, P value) in the adapted strain (P value of 0.1 in YO1-64, compared to 0.19 in the YO1-16 strain). The phospholipid composition of the adapted strain was not significantly altered; however, ergosterol content was reduced in YO1-64 from that in the YO1-16 strain. The asymmetrical distribution of phosphatidylethanolamine (PE) between two monolayers of plasma membrane was also changed, with PE becoming more exposed to the outer monolayer in the YO1-64 strain. The results of the present study suggest for the first time that changes in the status of membrane lipid phase and asymmetry could contribute to azole resistance in C. albicans.
Candida albicans is most frequently associated with human fungal infections. Widespread and prolonged usage of azoles in recent years has led to the rapid development of drug resistance in C. albicans, which has posed serious problems for its successful use in chemotherapy. C. albicans cells acquire multidrug resistance (MDR) during the course of a treatment; wherein continued exposure to drug(s) results in the development of multiple resistance mechanism(s) which include alterations of the target enzyme (lanosterol 14-α demethylase or ERG11), overexpression of drug extrusion pumps belonging to either the major facilitators or the ATP-binding cassette (ABC) superfamily (23, 35). There is evidence to suggest that impaired import of an antifungal agent which may involve membrane alterations through changes in the sterol and/or lipid content or formation of a biofilm may also be an important attribute of azole resistance (14, 23). In this context the importance of the membrane lipid phase (fluidity) in the overall scenario of MDR has only recently been appreciated. Human MDR1 (P-glycoprotein) activity has been found to be sensitive to the nature and the physical state of the lipid matrix (12). The intimate association of both P-glycoprotein (P-gp) and its hydrophobic substrates with the membrane matrix do suggest a possible role of membrane lipids in the modulation of drug binding activity of P-gp (30, 31, 33). It is observed that membrane cholesterol directly interacts with P-gp and affects drug binding to the membrane bilayer, which in turn could affect drug availability to P-gp (34). We have earlier demonstrated that CDR1 (ABC transporter of C. albicans)-mediated drug resistance was susceptible to changes in membrane fluidity (32). Interestingly, the physical state of membrane has also been shown to affect gene expression and signal transduction in yeast (25, 29).
The fact that most azole-resistant isolates are known to be associated with clinical failure justifies the need for a detailed analysis of the mechanisms of resistance in such isolates. The clonal nature of C. albicans makes it imperative that a matched set of susceptible and resistant isolates derived from a single strain be examined in characterizing the molecular mechanisms of resistance. The emergence of drug resistance in serial isolates of C. albicans from patients undergoing azole treatment in most cases was shown to develop from a previously more susceptible strain and was associated with the overexpression of certain MDR genes, such as CDR1, CDR2, CaMDR1, and ERG11 (35). There are also instances where overexpression of known MDR genes is not directly correlated with azole resistance, implying the contribution of yet other unknown mechanisms of resistance. It appears that azole resistance in C. albicans is a multifactorial phenomenon.
Although in vivo studies provide invaluable clues to the molecular changes associated with azole resistance, it is difficult to determine the time point when molecular changes result in a resistant phenotype. In several instances a mixed population of isolates is obtained (21). To overcome such problems, several groups have developed in vitro systems for the development of azole resistance by exposing C. albicans cells to increasing concentrations of fluconazole (1, 8). The present study was undertaken to delineate the cellular changes that may accompany the development of azole resistance in sequentially adapted in vitro fluconazole-resistant strains. The adaptation to fluconazole led to the overexpression of drug extrusion pump-encoding genes, particularly CDR1 and CDR2 and the gene for the azole target enzyme, ERG11. Of note was an increase in membrane fluidity, as was evident by fluorescence polarization measurements. Since the phospholipid composition of the adapted strain was not altered, the observed fluctuation in ergosterol content appeared to be responsible for the change in membrane order. The observed changes in membrane fluidity were also associated with the change in membrane lipid asymmetry of adapted strains. Our results suggest that membrane alterations associated with fluconazole resistance should be an important consideration in delineating the complex mechanism of drug resistance in C. albicans cells.
MATERIALS AND METHODS
Isolation of fluconazole-resistant yeast strains.
C. albicans strain YO1-16 used in this study is a clinical isolate which was a kind gift from NDDR, Ranbaxy, India. The MIC of this strain (YO1-16) was found to be 16 μg ml−1, and hence it is a susceptible dose-dependent (SDD) strain, as per NCCLS guidelines. Fluconazole-resistant mutants of this strain were obtained by serial passage (5 to 10 passages, each of 48-h duration) in increasing concentrations of fluconazole (2× MIC) from 32 to 64 μg ml−1 in RPMI media, resulting in sequentially adapted strains, YO1-32 and YO1-64, with MICs of 32 and 64 μg ml−1, respectively. MICs of the strains were determined using a broth microdilution method (11) and reading of the endpoints at 48 h. Cells were added to the microtiter plate at a final cell density of 2.5 × 103 cells ml−1. The MIC test end point was defined as the lowest drug concentration that gave >80% inhibition of growth compared with drug-free controls (MIC80). For all experimental studies the yeast cells were maintained on yeast extract-peptone-dextrose (YEPD) medium at 37°C.
Fluorescence polarization and sterol analysis.
Fluidity measurements were carried out as described earlier (2) by using the fluorescent probe 1,6-diphenyl-1,3,5-hexatriene (DPH) as a reporter. Briefly, cells were incubated with Zymolyase (100 U/g wet weight) at 37°C for 3 h with gentle shaking to remove the cell wall. Spheroplast preparation was monitored turbidometrically by checking the ability of 0.2% sodium dodecyl sulfate to lyse the enzyme-digested cells. Fluorescence polarization was measured at excitation and emission wavelengths of 360 and 426 nm, respectively. The measured fluorescence intensities were corrected for background fluorescence and the light scattering from the unlabeled sample. Sterols were extracted (2) and quantified as described previously by Arthington-Skaggs et al. (3).
Membrane lipid asymmetry measurements.
Fluorescamine, which specifically labels exposed aminophospholipids (9), was used for labeling of C. albicans resistant and susceptible strains. The protocol of labeling and quantitative estimation of asymmetry was strictly followed as described previously (10). Briefly, cells were harvested in mid-log phase by centrifuging at 3,000 rpm (Beckman model TJ-6) for 5 min at 4°C and washed two to three times with buffer A (100 mM potassium phosphate-5 mM EDTA, pH 7.5). The harvested cells (0.6 g wet weight) were resuspended in 5 ml of buffer B (100 mM potassium phosphate and 600 mM KCl, pH 8.2) and kept at 4°C with gentle swirling. Fluorescamine (15.6 mM) in dehydrated dimethyl sulfoxide was added dropwise to the cell suspension with constant gentle swirling. After 30 s the reaction was stopped by adding an equal volume of 1 M ammonia in 600 mM KCl. The cells were centrifuged and washed at 4°C three to four times till the color of the dye disappeared from the supernatant. Resolved phospholipids and derivatized phosphatidylethanolamine (PE) were scraped off from thin-layer chromatography (TLC) plates, and their phosphate content was estimated (17).
Analysis of mRNA in C. albicans adapted strains.
Total RNA isolation from C. albicans cells was done as described earlier (19). Northern analysis and transfers were done using standard laboratory protocols. RNA was isolated from strains grown in YEPD media in the absence of any antifungal drug. A 1.8-kb EcoRV fragment of CaMDR1 and DNA probes specific for CDR1 and CDR2 (PCR amplified from C. albicans ATCC 10261 genomic DNA as described previously [19]) were used as molecular probes for Northern analysis. Oligonucleotides (forward, 5′ TTCTAGAAGATCATAACTCA 3′, and reverse, 5′ GTTAATCGATCTAAGTAACA 3′) were used to amplify ERG11. The probes were partially sequenced to confirm their identities.
Glucose-induced rhodamine 6G (R6G) efflux.
Efflux of R6G was determined essentially using a protocol described by Maesaki et al. (22). Briefly, yeast cells were grown overnight in YEPD media. Cells (108) were transferred to 100 ml of fresh YEPD media and allowed to grow for 4 h; they were then resuspended in phosphate-buffered saline buffer at a cell density of 108 cells ml−1 and incubated at 37°C for 1 h in a shaker. R6G was then added at a final concentration of 10 μM and incubated for 25 min (till the dye showed a steady intracellular level of accumulation). One mole of glucose was then added to initiate R6G efflux. Samples of 1 ml in volume were withdrawn at the indicated time points and centrifuged at 9,000 × g for 2 min. The resulting supernatant was collected, and absorption was measured at 527 nm. Glucose-free controls were included in all experiments.
RESULTS AND DISCUSSION
Characterization of fluconazole-resistant strains.
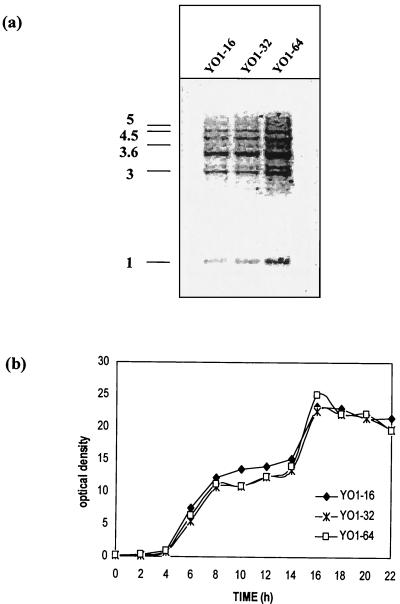
The C. albicans strains YO1-32 and YO1-64 were isolated by serial passage of a fluconazole-susceptible-dose-dependent clinical isolate, YO1-16, through medium containing fluconazole (see Materials and Methods). This was done to enhance the MIC threshold value for the YO1-16 strain to levels of 64 μg ml−1, which are generally considered acceptable values of clinical drug resistance in C. albicans (28). The adapted strain exhibited a twofold increase in the MIC of fluconazole that was determined by standard methods of antifungal susceptibility testing. The fluconazole resistance phenotype in the adapted strains (YO1-32 and YO1-64) was stable even after 10 to 20 transfers in drug-free media. In order to ensure stability of in vitro resistance, fluconazole susceptibility was routinely checked at each subsequent passage in drug-free media. The isogenicity of adapted strains was confirmed by using the CARE-2 fingerprinting probe (20) (Fig. 1a). The adapted strains (YO1-32 and YO1-64) showed no significant differences in growth compared with the progenitor strain, YO1-16 (Fig. 1b).
FIG. 1.
Isogenicity and growth patterns of fluconazole-susceptible and -resistant C. albicans strains. (a) CARE-2 hybridization pattern of EcoRI-digested chromosomal DNA of the C. albicans adapted strains. The positions of the molecular size markers (in kilobases) are shown on the left-hand side of the figure. (b) Comparison of growth curve of the fluconazole-susceptible and -resistant strains: the cells were grown in YEPD media at 37°C. Aliquot of cells was taken every 2 h till 22 h, and the optical density was measured at A600.
Susceptibilities of in vitro-adapted C. albicans strains to other drugs.
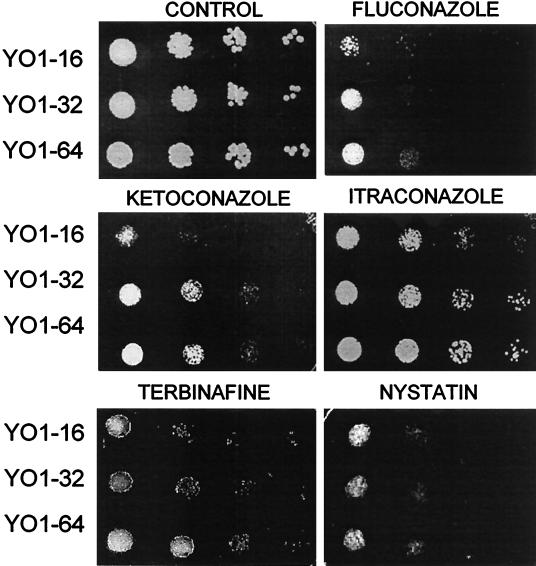
Earlier reports have shown that fluconazole-resistant clinical isolates are frequently cross-resistant to other azoles and can also be cross-resistant to polyenes (35). We therefore examined if adaptation to fluconazole was also associated with cross-resistance to other antifungals. Both spot assays and NCCLS microdilution method were employed for testing resistance to drugs such as azoles and allylamine (Fig. 2 and Table 1). Strains YO1-64 and YO1-32 were found to be more resistant to both ketoconazole and itraconazole than the progenitor cell. Besides azole drugs, the YO1-64 strain also exhibited significant resistance to terbinafine, an allylamine which inhibits the ERG1 gene product in the ergosterol biosynthetic pathway. Notably, although the differences in MICs obtained for different antifungals were small, they were consistently observed by repeated testing of isolates, which was also corroborated by spot assays. Thus, in spite of the fact that the adapted strains exhibited low levels of resistance to azoles (only fourfold-higher MICs for fluconazole), this difference in susceptibilities appeared to be sufficient to result in the development of the MDR phenotype exhibited by these cells.
FIG. 2.
Susceptibilities of adapted strains to antifungal agents. The yeast cells were grown overnight on YEPD plates at 37°C. Cells were then suspended in normal saline to an optical density of 0.1 (A600). Five microliters of fivefold serial dilutions of each yeast culture were spotted onto YEPD plates in the absence (control) and presence of the indicated drugs: fluconazole (8 μg ml−1), ketoconazole (0.16 μg ml−1), itraconazole (0.16 μg ml−1), terbinafine (12.5 μg ml−1), and nystatin (1 μg ml−1). Growth differences were recorded following incubation of the plates for 48 h at 37°C. Growth was not affected by the presence of the solvents used for the drugs.
TABLE 1.
Azole susceptibility of adapted C. albicans strainsa
| Strain | MICb (μg ml−1) of:
|
Source | ||||
|---|---|---|---|---|---|---|
| FLC | ITRA | KETO | TERB | NYS | ||
| YO1-16 | 16 | 0.125 | 0.03125 | 0.5 | 5 | NDDR, Ranbaxy India |
| YO1-32 | 32 | 0.25 | 0.0625 | 1 | 5 | This study |
| YO1-64 | 64 | 0.25 | 0.0625 | 2 | 5 | This study |
MICs were determined by a microdilution method and by reading end points after a 48-h incubation of plates at 37°C as described in Materials and Methods. FLC, fluconazole; ITRA, itraconazole; KETO, ketoconazole; TERB, terbinafine; NYS, nystatin.
MIC test end point was defined as the lowest drug concentration that gave >80% inhibition of growth.
Adapted strains exhibit elevated levels of membrane fluidity.
Since the intracellular drug level is dependent not only on active drug efflux but also on the rate of its drug import (35), the status of the membrane lipid matrix could be an important determinant in the development of drug resistance. In the following experiments this aspect was examined. Spectral analysis and subsequent quantification of extracted sterols (3) revealed that the YO1-16 and YO1-32 strains have similar levels of ergosterol. However, the ergosterol content was found to be reduced in strain YO1-64 strain (see Table 2). In many instances reduced ergosterol content is associated with increased resistance to polyenes like nystatin (16), which exerts it antifungal effect by binding to the membrane ergosterol. However, our results show that the adapted strains remained susceptible to nystatin (Table 1; Fig. 2). This is not unusual, since prior studies by members of our group and others have shown that binding of polyenes to membranes is not solely dependent upon sterols, since other membrane components and factors, such as fatty acids and phospholipids, could contribute to their action (5, 24).
TABLE 2.
Phospholipid and sterol composition of fluconazole-susceptible and -resistant strains
| Strain | Phospholipid content (%) (mean ± SD)a
|
ERGb | ||||||
|---|---|---|---|---|---|---|---|---|
| SPH | PI | PS | PC | PE | CL | PA | ||
| YO1-16 | 1.30 ± 1.14 | 13.73 ± 5.35 | 14.52 ± 0.44 | 45.67 ± 4.32 | 16.07 ± 3.25 | 5.80 ± 0.72 | 2.11 ± 0.78 | 1.2 ± 0.06 |
| YO1-32 | 2.33 ± 0.49 | 12.87 ± 3.03 | 13.13 ± 2.14 | 44.21 ± 4.50 | 15.54 ± 1.77 | 8.59 ± 1.89 | 2.48 ± 1.41 | 1.26 ± 0.05 |
| YO1-64 | 4.38 ± 1.24 | 13.5 ± 1.42 | 12.32 ± 2.02 | 40.75 ± 6.78 | 17.93 ± 2.07 | 8.93 ± 2.25 | 1.95 ± 0.48 | 0.74 ± 0.06 |
Phospholipid content of fluconazole-susceptible and -resistant C. albicans cells is expressed as a percentage of total lipids. Values represent means from at least six determinations. Constituent phospholipids have been abbreviated as follows: SPH, sphingolipid; PI, phosphatidylinositol; PS, phosphatidylserine; PC, phosphatidylcholine; PE, phosphatidylethanolamine; CL, cardiolipin; PA, phosphatidic acid.
Mean ergosterol content (ERG) of the cells is expressed as a percentage of the wet weight of the cell ± the standard deviations of the mean of three sets of experiments (3).
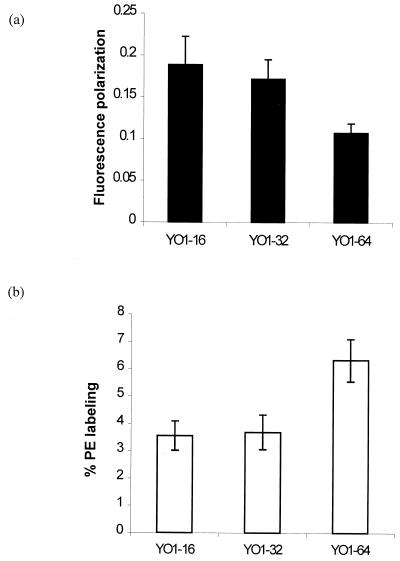
The membrane order (fluidity) of the adapted strain was determined by using the fluorescent probe DPH. Interestingly, fluorescence polarization measurements of the adapted strain YO1-64 showed enhanced fluidity (low P value) compared to its susceptible counterpart, in the following order: YO1-64 had greater fluidity than YO1-32, which had greater fluidity than YO1-16 (Fig. 3a). The phospholipid composition of the adapted cells did not contribute to observed change in fluidity, since it was not significantly altered between resistant and susceptible strains except for sphingolipid content, which was higher in resistant strains than in susceptible isolates (15) (Table 2). Of note, the observed enhanced fluidity of the YO1-64 strain was probably associated with decreased levels of ergosterol. The decreased level of ergosterol in the YO1-64 strain could also reflect a decrease in the activity of Δ5,6desaturase enzyme, which is suppressed when 14α-demethylase (target enzyme of fluconazole) activity is reduced either pharmacologically (by azoles) or genetically (by gene disruption) (26, 35). Whether desaturation of fatty acids or intermediates of the ergosterol biosynthetic pathway may also contribute to observed elevated levels of membrane fluidity remains to be analyzed.
FIG. 3.
(a) Fluorescence polarization measurements in fluconazole-susceptible and -resistant strains. Fluorescence polarization measurements were carried out using fluorescent probe DPH, as described in Materials and Methods, at excitation and emission wavelengths of 360 and 426 nm, respectively. Each experiment was done in triplicate, and the values represent means ± standard deviations. (b) Percentage labeling of phosphatidylethanolamine (PE) in plasma membrane of C. albicans cells with fluorescamine. Cells were labeled with fluorescamine as described in Materials and Methods. After labeling, cells were washed and lipids were extracted. The percentage of derivatized PE was determined as described in Materials and Methods. The results shown are the means of more than six independent experiments ± standard deviations.
Adapted strain shows change in membrane phospholipid asymmetry.
We have earlier demonstrated that the membrane phospholipid translocation between the two monolayers of the lipid bilayer of the plasma membrane of C. albicans is maintained by an energy-dependent process mediated by the ABC transporter protein, e.g., Cdr1p (10). Keeping in view that Cdr1p functioning is also sensitive to alterations in membrane fluidity (32), we examined whether adapted resistant strains will display any change with regard to asymmetrical distribution of phospholipids. The fluorescent dye fluorescamine, which labels exposed aminophospholipids, was used to quantitate aminophospholipids. Since phosphatidylserine is localized predominantly in the cytoplasmic leaflet of the lipid bilayer, the dye could label only PE, which is also present in the outer monolayer (10). The labeling experiments revealed that the percentage of exposed PE was highest in the YO1-64 strain (Fig. 3b), which also showed maximum expression of CDR1 (discussed below). No statistically significant increase in percentage labeling of PE was, however, observed in strain YO1-32, which also showed low-level expression of CDR genes. Interestingly, the membranes of strain YO1-64 were more fluid, which may be an important factor regulating phospholipid translocation mediated by overexpressed CDR1. The involvement of Cdr1p in phospholipid translocation was earlier demonstrated from the fact that an increased amount of PE in the outer leaflet of the plasma membrane of the mycelial form correlates well with CDR1 expression, which is also more pronounced in mycelia than in the bud form of C. albicans (10). Of note, the exposed PE was also found to be decreased in a CDR1 disruptant strain. Thus, there appears to be a causal relationship between CDR1 overexpression and phospholipid translocation.
Molecular changes accompanying azole resistance.
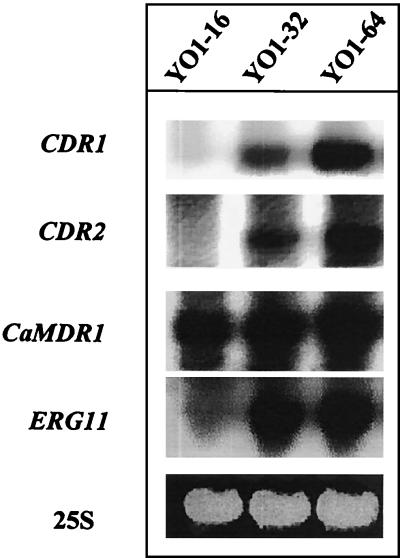
As mentioned, in many instances overexpression of genes like CDR1/2 and CaMDR1 in clinical isolates of C. albicans have been associated with fluconazole resistance (19, 35). We compared the expression levels of potential MDR genes of C. albicans, such as those for CDR1, CDR2, CaMDR1, and the azole target enzyme, ERG11, in these strains. The SDD strain YO1-16 did not show any detectable levels of CDR1 or CDR2 mRNA (Fig. 4). In contrast, a two-step induction of CDR1 and CDR2 genes was observed which was highest in the azole-resistant strain, YO1-64 (Fig. 4). While the YO1-16 strain showed very low levels of ERG11, both YO1-32 and YO1-64, for which MICs are higher, showed up-regulation of ERG11. There was no significant difference in CaMDR1 expression between these isolates. It should be pointed out that while the expression of ERG11 is detected in most of the susceptible isolates, the SDD strain YO1-16 used in this study showed only low levels of ERG11 transcript. This is not unusual, since earlier studies have included similar observations (13, 21).
FIG. 4.
Northern blots of total RNA from fluconazole-susceptible and -resistant C. albicans strains. RNA was isolated from cells grown logarithmically and hybridized with probes specific for CDR1, CDR2, MDR1, and ERG11. Hybridizations were performed as described in Materials and Methods. The bottom panel shows the corresponding gel load (25S rRNA).
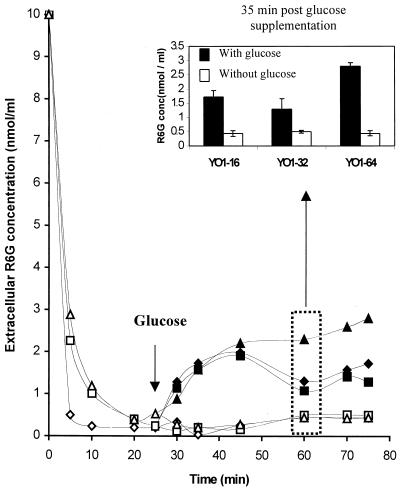
Overexpression of CDR1/2 genes in clinical isolates of C. albicans is known to render a cell resistant to many different azoles, while overexpression of CaMDR1 appears to be specific for fluconazole resistance and is not associated with resistance to other azoles (35). Therefore, the cross-resistance to azoles of adapted C. albicans cells would suggest that it could be predominantly due to their decreased accumulation of drug mediated by overexpressed efflux pumps, such as Cdr1p and Cdr2p (Fig. 2). We checked the efflux of R6G, a well-known fluorescent compound that is transported by a wide variety of MDR proteins (6, 7). In most of the studies, the net intracellular accumulation of the drug is taken as an indirect criterion of efflux measurements, but such measurements do not exclude the contribution of drug import by diffusion. Therefore, in order to minimize the contribution of drug import, we used R6G and studied its efflux in energy-starved cells. Figure 5 depicts a rapid drop of R6G fluorescence in the supernatant, which became steady after 25 min. The decrease in R6G fluorescence in the supernatant of energy-starved cells was due to its rapid diffusion into the cells. When the efflux was initiated by energy supplementation (addition of glucose), there was a steady increase in R6G fluorescence in the supernatant, which was due to its efflux from the cells mediated by drug extrusion pumps like CDR1 and CDR2. As can be seen in Fig. 5, the adapted strain could elicit an increased glucose-induced drug efflux that was more pronounced in YO1-64 than in the parent strain. However, this difference became more apparent at later time points of glucose supplementation, when the difference in the percentage efflux of R6G in YO1-64 was almost twofold compared to YO1-16 strain (Fig. 5, inset). It should be pointed out that at early time points all the strains had similar levels of R6G efflux. Of note, no R6G efflux was observed when these strains were maintained in the absence of glucose. Thus, the observed enhanced energy-dependent R6G efflux in YO1-64 compared to YO1-32 strains probably contributes to resistance.
FIG. 5.
R6G uptake and glucose-induced R6G efflux from fluconazole-susceptible and -resistant strains. The assay was performed essentially as described in Materials and Methods (22). Cells from YO1-16 (⋄), YO1-32 (□), and YO1-64 (▵) were incubated with 10 μM R6G at 37°C. One mole of glucose was added after a 25-min incubation in glucose-free phosphate-buffered saline. The corresponding filled symbols represent the extracellular concentrations of R6G in the presence of glucose. The inset shows R6G efflux at 35 min post-glucose induction, which gives a comparison with controls incubated without glucose. Each bar indicates the standard deviations of mean of four sets of experiments.
Taken together, our results demonstrate that in vitro drug resistance exhibited by a fluconazole-adapted series of isolates correlates well with that observed in vivo in a clinical situation wherein a stepwise increase in acquisition of MDR traits is observed. Interestingly, the gradual onset of resistance mechanisms in the sequentially fluconazole-adapted isolates of C. albicans is associated with changes in membrane lipid fluidity and asymmetry. That the fluctuation in lipid environment could also affect drug susceptibilities is apparent from several studies wherein both the import and export of drugs are shown to be affected by membrane perturbations (12, 18, 27, 32). Recent results from our laboratory show that azole-resistant clinical isolates of C. albicans possess altered membrane fluidity (Prasad et al., unpublished observations). Of interest, resistance to platelet microbicidal proteins by Staphylococcus aureus was also shown to be associated with variation in membrane fluidity due to elevated levels of unsaturated membrane lipids (4). In view of the accumulating evidence, it is apparent that the changes in the membrane lipid environment are associated with drug resistance, and therefore, the interplay between the physical state of membrane lipids and azole resistance mechanisms in C. albicans probably requires a closer look.
Acknowledgments
We are thankful to Pfizer Ltd., Sandwich, Kent, United Kingdom for providing fluconazole. Itraconazole and ketoconazole were kind gifts from the Janssen Research Foundation, Beerse, Belgium. The CARE-2 probe was a kind gift from B. A. Lasker. We are grateful to R. N. Saini for photographic assistance.
The work presented in this paper has been supported in parts by grants to R.P. from the Department of Biotechnology (DBT-BT/PRO798/HRD20/8/98 BRPC), Department of Science and Technology (SP/SO/D57/97), Council of Scientific and Industrial Research (CSIR) (60(0028)/98-EMR-II), India. A.K. and K.M. acknowledge the fellowship award from the University Grants Commission and CSIR, respectively.
REFERENCES
- 1.Albertson, G. D., M. Niimi, R. D. Cannon, and H. F. Jenkinson. 1996. Multiple efflux mechanisms are involved in Candida albicans fluconazole resistance. Antimicrob. Agents Chemother. 40:2835-2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ansari, S., P. Gupta, S. K. Mahanty, and R. Prasad. 1993. The uptake of amino acids by erg mutants of Candida albicans. J. Med. Vet. Mycol. 31:377-386. [Google Scholar]
- 3.Arthington-Skaggs, B. A., H. Jradi, T. Desai, and C. J. Morrison. 1999. Quantitation of ergosterol content: novel method for determination of fluconazole susceptibility of Candida albicans. J. Clin. Microbiol. 37:3332-3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bayer, A. S., R. Prasad, J. Chandra, A. Koul, M. Smriti, A. Varma, R. A. Skurray, N. Firth, M. H. Brown, S. Koo, and M. R. Yeaman. 2000. In vitro resistance of Staphylococcus aureus to thrombin-induced platelet microbicidal protein is associated with alterations in cytoplasmic membrane fluidity. Infect. Immun. 68:3548-3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolard, J., and J. Milhaud. 1996. Interaction of anti-Candida amphotericin B (and other polyene antibiotics) with lipids, p. 253-274. In R. Prasad and M. A. Ghannoum (ed.), Lipids of pathogenic fungi. CRC Press, Boca Raton, Fla.
- 6.Canitrot, Y., S. Lahmy, J. J. Buquen, D. Canitrot, and D. Lautier. 1996. Functional study of multidrug resistance with fluorescent dyes. Limits of the assay for low levels of resistance and application in clinical samples. Cancer Lett. 106:59-68. [DOI] [PubMed] [Google Scholar]
- 7.Clark, F. S., T. Parkinson, C. A. Hitchcock, and N. A. R. Gow. 1996. Correlation between Rhodamine 123 accumulation and azole sensitivity in Candida species: possible role for drug efflux in drug resistance. Antimicrob. Agents Chemother. 40:419-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cowen, L. E., D. Sanglard, D. Calabrese, C. Sirjusingh, J. B. Anderson, and L. M. Kohn. 2000. Evolution of drug resistance in experimental populations of Candida albicans. J. Bacteriol. 182:1515-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diaz, C., and A. J. Schroit. 1996. Role of translocases in the generation of phosphatidylserine asymmetry. J. Membr. Biol. 151:1-9. [DOI] [PubMed] [Google Scholar]
- 10.Dogra, S., S. Krishnamurthy, V. Gupta, B. L. Dixit, C. M. Gupta, D. Sanglard, and R. Prasad. 1999. Asymmetric distribution of phosphatidylethanolamine in C. albicans: possible mediation by CDR1, a multidrug transporter belonging to ATP binding cassette (ABC) superfamily. Yeast 15:111-121. [DOI] [PubMed] [Google Scholar]
- 11.Espinell-Ingroff, A., F. Barchiesi, K. C. Hazen, J. V. Martinez-Suarez, and G. Scalise. 1998. Standardization of antifungal susceptibility testing and clinical relevance. Med. Mycol. 36:68-78. [PubMed] [Google Scholar]
- 12.Ferte, J. 2000. Analysis of the tangled relationships between P-glycoprotein-mediated multidrug resistance and the lipid phase of the cell membrane. Eur. J. Biochem. 267:277-294. [DOI] [PubMed] [Google Scholar]
- 13.Franz, R., S. L. Kelly, D. C. Lamb, D. E. Kelly, M. Ruhnke, and J. Morschhauser. 1998. Multiple molecular mechanisms contribute to a stepwise development of fluconazole resistance in clinical Candida albicans strains. Antimicrob. Agents Chemother. 42:3065-3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hawser, S. P., and L. J. Douglas. 1995. Resistance of Candida albicans biofilms to antifungal agents in vitro. Antimicrob. Agents Chemother. 39:2128-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hitchcock, C. A., K. Barrett-Bee, and N. J. Russel. 1986. The lipid composition of azole-sensitive and azole-resistant strains of Candida albicans. J. Gen. Microbiol. 132:2421-2431. [DOI] [PubMed] [Google Scholar]
- 16.Hitchcock, C. A., K. J. Barrett-Bee, and N. J. Russel. 1987. The lipid composition and permeability to azole of an azole- and polyene-resistant mutant of Candida albicans. J. Med. Vet. Mycol. 25:29-37. [DOI] [PubMed] [Google Scholar]
- 17.Ibrahim, A. S., and M. A. Ghannoum. 1996. Chromatographic analysis of lipids, p. 52-79. In R. Prasad (ed.), Manual on membrane lipids. Springer Verlag, Berlin, Germany.
- 18.Kaur, R., and A. K. Bachhawat. 1999. The yeast multidrug resistance pump, Pdr5p, confers reduced drug resistance in erg mutants of Saccharomyces cerevisiae. Microbiology 145:809-818. [DOI] [PubMed] [Google Scholar]
- 19.Krishnamurthy, S., V. Gupta, R. Prasad, S. L. Panwar, and R. Prasad. 1998. Expression of CDR1, a multidrug resistance gene of Candida albicans: in vitro transcriptional activation by heat shock, drugs and human steroid hormones. FEMS Microbiol. Lett. 160:191-197. [DOI] [PubMed] [Google Scholar]
- 20.Lasker, B. A., L. S. Page, T. J. Lott, and G. S. Kobayashi. 1992. Isolation, characterisation, and sequencing of Candida albicans repetitive element 2. Gene 116:51-57. [DOI] [PubMed] [Google Scholar]
- 21.Lopez-Ribot, J. L., R. K. McAtee, S. Perea, W. R. Kirkpatrick, M. G. Rinaldi, and T. F. Patterson. 1999. Multiple resistant phenotypes of Candida albicans coexist during episodes of oropharyngeal candidiasis in human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 43:1621-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maesaki, S., P. Marichal, H. V. Bossche, D. Sanglard, and S. Kohno. 1999. Rhodamine 6G efflux for the detection of CDR1-overexpressing azole-resistant Candida albicans strains. J. Antimicrob. Chemother. 44:27-31. [DOI] [PubMed] [Google Scholar]
- 23.Marichal, P. 1999. Mechanisms of resistance to azole antifungal compounds. Curr. Opin. Anti-infective Investig. Drugs 1:318-333. [Google Scholar]
- 24.Mishra, P., J. Bolard, and R. Prasad. 1992. Emerging role of lipids of Candida albicans, a pathogenic dimorphic yeast. Biochim. Biophys. Acta 1127:1-14. [DOI] [PubMed] [Google Scholar]
- 25.Moskvina, E., E.-M. Imre, and H. Ruis. 1999. Stress factors acting at the level of plasma membrane induce transcription via the stress response element (STRE) of the yeast Saccharomyces cerevisiae. Mol. Microbiol. 32:1263-1272. [DOI] [PubMed] [Google Scholar]
- 26.Prasad, R., S. L. Panwar, and S. Krishnamurthy. 2002. Drug resistance mechanisms of human pathogenic fungi, p. 601-631. In R. A. Calderone and R. L. Cihlar (ed.), Fungal pathogenesis: principles and clinical applications. Marcel Dekker, New York, N.Y.
- 27.Regev, R., Y. G. Assaraf, and G. D. Eytan. 1999. Membrane fluidization by ether, other anesthetics, and certain agents abolishes P-glycoprotein ATPase activity and modulates efflux from multidrug-resistant cells. Eur. J. Biochem. 259:18-24. [DOI] [PubMed] [Google Scholar]
- 28.Rex, J. H., M. A. Pfaller, J. N. Galgiani, M. S. Bartlett, A. Espinel-Ingroff, M. A. Ghannoum, M. Lancaster, F. C. Odds, M. G. Rinaldi, T. J. Walsh, and A. L. Barry. 1997. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole and Candida infections. Clin. Infect. Dis. 24:235-247. [DOI] [PubMed] [Google Scholar]
- 29.Ruis, H., and C. Schuller. 1995. Stress signaling in yeast. BioEssays 17:959-965. [DOI] [PubMed] [Google Scholar]
- 30.Saeki, T., A. M. Shimabuku, Y. Azuma, Y. Shibano, T. Komano, and T. Ueda. 1991. Expression of human P-glycoprotein in yeast cells: effects of membrane component sterols on the activity of P-glycoprotein. Agric. Biol. Chem. 55:1859-1865. [PubMed] [Google Scholar]
- 31.Sharom, F. J. 1996. The P-glycoprotein multidrug transporter: interactions with membrane lipids, and their modulation of activity. Biochem. Soc. Trans. 25:1088-1096. [DOI] [PubMed] [Google Scholar]
- 32.Smriti, S. Krishnamurthy, and R. Prasad. 1999. Membrane fluidity affects functions of Cdr1p, a multidrug ABC transporter of Candida albicans. FEMS Microbiol. Lett. 173:475-481. [DOI] [PubMed] [Google Scholar]
- 33.Urbatsch, I. L., and A. E. Senior. 1995. Effects of lipids on ATPase activity of purified Chinese hamster P-glycoprotein. Arch. Biochem. Biophys. 316:135-140. [DOI] [PubMed] [Google Scholar]
- 34.Wang, E., C. N. Casciano, R. P. Clement, and W. W. Johnson. 2000. Cholesterol interaction with the daunorubicin binding site of P-glycoprotein. Biochem. Biophys. Res. Commun. 276:909-916. [DOI] [PubMed] [Google Scholar]
- 35.White, T. C., K. A. Marr, and R. A. Bowden. 1998. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin. Microbiol. Rev. 11:382-402. [DOI] [PMC free article] [PubMed] [Google Scholar]