Abstract
Antibiotic susceptibility testing by disk diffusion of a Chryseobacterium gleum isolate, strain CIP 103039, showed a typical synergy image between clavulanic acid and expanded-spectrum cephalosporins. Shotgun cloning gave a recombinant plasmid in Escherichia coli that produced a β-lactamase, CGA-1, with a pI value of 8.9 that conferred resistance to most penicillins (except ureidopenicillins) and narrow-spectrum cephalosporins and an intermediate susceptibility to expanded-spectrum cephalosporins and aztreonam. The CGA-1 amino acid sequence shared only 60% amino acid identity with CME-1 and CME-2 from Chryseobacterium meningosepticum, the most closely related β-lactamases. CGA-1 was very likely chromosome encoded. It is a novel member of the PER subgroup of Ambler class A β-lactamases (Bush functional group 2be).
The Flavobacterium genus, including unsporulate gram-negative rods that produced yellow pigments, was identified in 1923. It underwent several classification changes. Flavobacterium IIb includes bacteria that exhibit a deep yellow pigment and are negative for DNase and for lactose oxidation (9, 10). Flavobacterium IIb has been described as a human pathogen responsible for bacteremia, meningitis, and nosocomial infections (18). This group showed genotypic heterogeneity (22) and has been reclassified as Flavobacterium indologenes and Flavobacterium gleum (9, 25). In 1990, both F. indologenes and F. gleum were genotypically and phenotypically differentiated (26). Finally in 1994, Vandamme et al. proposed the novel genus Chryseobacterium that included Chryseobacterium meningosepticum, Chryseobacterium indologenes, Chryseobacterium indoltheticum, Chryseobacterium scophthalmum, and Chryseobacterium gleum (23).
Most of the C. gleum isolates reported in the literature have a clinical origin (sputum wound swab or high vaginal swab) (25), but at least in the early clinical reports, they had not been differentiated from the other Chryseobacterium sp. isolates.
Two variants of a chromosomally encoded Ambler class A extended-spectrum β-lactamase (ESBL) (CME-1 and CME-2) that share 98% amino acid identity have recently been reported from C. meningosepticum isolates (3, 16) while no serine β-lactamases have been reported from C. indologenes, in which only metallo-β-lactamases were found (2). CME-like enzymes confer a high level of resistance to β-lactams, including expanded-spectrum cephalosporins. Their activities are inhibited by clavulanic acid, tazobactam, imipenem, cefoxitin, and moxalactam (3). These enzymes are classified within the Bush functional group 2be (5).
Antibiotic susceptibility testing by disk diffusion gave a synergy image between clavulanic acid- and ceftazidime-containing disks with a C. gleum reference strain. Thus, a genetic and biochemical characterization of the β-lactamase content of this strain was conducted and permitted the identification of a novel Ambler class A ESBL that was poorly related to any known class A enzymes.
MATERIALS AND METHODS
Bacterial strains.
The C. gleum reference strain CIP 103039 used in this study was from the Pasteur Institute strain collection. Escherichia coli DH10B and nalidixic acid-resistant E. coli JM109 were used for cloning and conjugation assays, respectively. All strains were stored at −70°C in Trypticase soy (TS) broth (Becton Dickinson, Le Pont-de-Claix, France) supplemented with 15% glycerol until testing. β-Lactamase-producing strains were screened with nitrocefin-containing disks (Biomérieux, Marcy l'Étoile, France).
Antimicrobial agents and MIC determinations.
The antimicrobial agents were obtained in the form of standard laboratory powders and were used immediately after their solubilization. The agents and their sources have been described elsewhere (13). Antibiotic disks were used for routine antibiograms (Sanofi Diagnostics Pasteur, Marnes-la-Coquette, France).
MICs were determined by an agar dilution technique on Mueller-Hinton agar (Sanofi Diagnostics Pasteur) with an inoculum of 104 CFU per spot (12). The plates were incubated at 35°C for 18 h. The β-lactam MICs were determined with the drug either alone or in combination with a fixed concentration of clavulanic acid (2 μg/ml) or tazobactam (4 μg/ml).
Cloning experiments and analysis of recombinant plasmids.
Genomic DNA from C. gleum CIP 103039 was extracted as described previously (13). All enzymes used in cloning experiments were from Amersham Pharmacia Biotech (Orsay, France). Fragments from genomic DNA partially digested with Sau3AI were ligated into BamHI-restricted phagemid pBK-CMV (Stratagene, Amsterdam, The Netherlands) as previously reported (3). Recombinant plasmids were transformed by electroporation (Gene Pulser II; Bio-Rad, Ivry sur Seine, France) into E. coli DH10B electrocompetent cells (GibcoBRL, Life Technologies, Cergy Pontoise, France). Antibiotic-resistant colonies were selected on TS agar plates containing ceftazidime (1 μg/ml) and kanamycin (30 μg/ml).
Recombinant plasmid DNA was obtained from 100-ml TS broth cultures grown overnight in the presence of ceftazidime (1 μg/ml) at 37°C. Plasmid DNAs were extracted and purified with a plasmid DNA maxi kit (Qiagen, Courtaboeuf, France).
Conjugation assays, plasmid content, and hybridization experiments.
The direct transfer of resistance genes into nalidixic acid-resistant E. coli JM109 was attempted by liquid and solid conjugation assays at 30 and 37°C (15). Transconjugants were selected on TS agar plates containing nalidixic acid (100 μg/ml) and ceftazidime (1 μg/ml). Extraction of plasmid DNA from C. gleum CIP 103039 was attempted by two different methods (8, 11). Southern hybridizations were performed as described previously (17) with whole-cell DNAs of C. gleum CIP 103039 by using the enhanced chemiluminescence nonradioactive labeling and detection kit (Amersham Pharmacia Biotech) with a PCR-obtained probe with internal primers for blaCGA-1 (cga-a, 5′-CAGCATTTTCATTGGCTCAG-3′; cga-b, 5′-CCTGCAGTTCACTGCATC-3′) consisting of a 796-bp internal fragment of blaCGA-1.
β-Lactamase purification, isoelectric focusing (IEF) analysis, relative molecular mass determination, and induction study.
A culture of E. coli DH10B harboring recombinant plasmid pCGA-1 was grown overnight at 37°C in 8 liters of TS broth containing amoxicillin (50 μg/ml). Bacterial suspensions were pelleted, resuspended in 60 ml of 20 mM Tris-HCl buffer (pH 8.0), and incubated for 30 min at 4°C with lysozyme (1 mg/ml) and DNase (1 μg/ml) with magnetic stirring. The pellet was then disrupted by sonication (three times at 20 W for 30 s with a Vibra Cell 75022 Phospholyser [Bioblock, Illkirch, France]) and centrifuged for 1 h at 48,000 × g at 4°C. The supernatant was then ultracentrifuged at 100,000 × g for 1 h at 4°C, and the supernatant was dialyzed overnight against phosphate buffer (50 mM, pH 7.0).
This extract was loaded onto a preequilibrated Q-Sepharose column (Amersham Pharmacia Biotech) in a 20 mM Tris-HCl buffer (pH 8.0). The fractions with the highest β-lactamase activities, as determined qualitatively by the nitrocefin test (Oxoid, Dardilly, France), were recovered in the flowthrough and subsequently dialyzed overnight against 50 mM sodium phosphate buffer (pH 7.0). The β-lactamase extract was then loaded onto an S-Sepharose column preequilibrated with the same buffer and eluted with a linear NaCl gradient (0 to 500 mM) in the same phosphate buffer. The fractions containing the highest β-lactamase activities (eluted at 200 mM NaCl) were pooled and dialyzed against 50 mM phosphate buffer (pH 7.0) containing 150 mM NaCl and concentrated with a Vivaspin 10,000 column (Sartorius, Göttingen, Germany). This concentrated β-lactamase extract was loaded onto a 1.6- by 47-cm gel filtration column (Amersham Pharmacia) packed with Superdex 75 (Amersham Pharmacia) equilibrated with 50 mM phosphate buffer (pH 7.0) containing 150 mM NaCl. The fraction containing the β-lactamase activity was then dialyzed overnight against 50 mM phosphate buffer (pH 7.0).
Specific activities of crude β-lactamase extract and purified enzyme were determined as previously reported (3) with 100 μM cephalothin as the substrate. The purity of the enzyme was estimated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and its relative molecular mass was determined by gel filtration.
Purified enzyme and β-lactamase crude extract from a 100-ml culture of C. gleum were subjected to analytical IEF as previously described (3).
To test the inducibility of β-lactamase production in a C. gleum isolate, induction experiments were performed with cefoxitin (1.5 and 10 μg/ml) as the inducer as previously described (14). Briefly, overnight cultures were diluted 1:10 and were grown for 1 h and 30 min in a preincubated TS broth on a rotating shaker at 37°C. Then the cultures were grown for an additional 2 h in the presence of the inducer. One unit of enzyme activity was defined as the activity which hydrolyzed 1 μmol of cephalothin per min. The total protein content was measured with bovine albumin as the standard (Bio-Rad DC protein assay kit).
Kinetic parameters.
The purified β-lactamase CGA-1 was used for kinetic measurements performed at 30°C in 100 mM sodium phosphate (pH 7.0). The rates of hydrolysis were determined with a spectrophotometer ULTROSPEC 2000 (Amersham Pharmacia Biotech). The wavelengths and absorption coefficients of β-lactams were as reported previously (15).
Km and kcat values were determined by analyzing the β-lactam hydrolysis under initial rate conditions by using the Eadie-Hofstee linearization of the Michaelis-Menten equation.
Various concentrations of clavulanic acid, tazobactam, cefoxitin, and imipenem were preincubated with the enzyme for 3 min at 30°C before the rate of benzylpenicillin (100 μM) hydrolysis was tested. The 50% inhibitory concentrations (IC50s) of these inhibitors were determined as the concentrations of these inhibitors that inhibited hydrolytic activity by 50%. Results were expressed in micromolar units.
DNA sequencing and protein analysis.
Both strands of the cloned DNA fragment of recombinant plasmid pCGA-1 were sequenced with an Applied Biosystems sequencer (ABI 377). The nucleotide sequence and the deduced protein sequence were analyzed with software available over the internet at the National Center for Biotechnology Information website (www.ncbi.nlm.nih.gov). Multiple-protein-sequence alignments were carried out with the program ClustalW available over the internet (www.biomed.pasteur.fr). A dendrogram was performed by a parsimony method with the phylogeny package PAUP (Phylogenetic Analysis Using Parsimony) version 3.0 (20).
Nucleotide sequence accession number.
The nucleotide sequence data reported in this paper will appear in the GenBank nucleotide database under the accession no. AF339733.
RESULTS AND DISCUSSION
Cloning experiments and antibiotic susceptibility testing.
Genomic DNA from C. gleum CIP 103039 that had been partially digested with Sau3AI endonuclease was cloned into the BamHI site of pBK-CMV. Eighteen recombinant E. coli DH10B strains were obtained after selection on ceftazidime- and kanamycin-containing MH agar plates. Preliminary antibiotic susceptibility testing showed that these E. coli strains displayed a typical clavulanic acid-inhibited ESBL phenotype. The insert sizes of recombinant plasmids varied from 1.4 to 10 kb. Plasmid pCGA-1, which possessed a 1.4-kb insert, was retained for further analysis.
The MICs of β-lactams for C. gleum CIP 103039 indicated that it was resistant to amino- and carboxy-penicillins and narrow-spectrum cephalosporins and of intermediate susceptibility to cefotaxime and aztreonam. The MIC of cefotaxime was higher than that of ceftazidime (Table 1) as opposed to what had been reported for a CME-2 β-lactamase-producing C. meningosepticum isolate (3). C. gleum CIP 103039 also showed a decreased susceptibility to ureidopenicillins and carbapenems (Table 1). The MICs of β-lactams for E. coli DH10B(pCGA-1) mirrored those for C. gleum CIP 103039 but to a lesser extent, except for amoxicillin. Thus, the cloned β-lactamase gene could not alone explain the β-lactam resistance phenotype observed for C. gleum CIP 103109. The MICs of β-lactams were significantly lowered by the addition of clavulanic acid and tazobactam as reported for C. meningosepticum (3).
TABLE 1.
MICs of β-lactams for C. gleum CIP 103039, E. coli DH10B(pCGA-1), and E. coli DH10B
| β-Lactam(s)a | MIC (μg/ml) for:
|
||
|---|---|---|---|
| C. gleum CIP 103039 | E. coli DH10B(pCGA-1) | E. coli DH10B | |
| Amoxicillin | 256 | 512 | 2 |
| Amoxicillin-CLA | 256 | 16 | 2 |
| Amoxicillin-TZB | 256 | 16 | 2 |
| Ticarcillin | 256 | 64 | 2 |
| Ticarcillin-CLA | 256 | 4 | 2 |
| Ticarcillin-TZB | 256 | 8 | 2 |
| Piperacillin | 8 | 8 | 1 |
| Piperacillin-TZB | 4 | 2 | 1 |
| Cephalothin | 256 | 64 | 2 |
| Ceftazidime | 4 | 16 | 0.06 |
| Ceftazidime-CLA | 0.5 | 1 | 0.06 |
| Ceftazidime-TZB | 0.5 | 0.5 | 0.06 |
| Cefotaxime | 32 | 0.5 | 0.06 |
| Cefotaxime-CLA | 32 | 0.06 | 0.06 |
| Cefotaxime-TZB | 32 | 0.06 | 0.06 |
| Cefepime | 1 | 0.5 | 0.06 |
| Cefpirome | 2 | 0.5 | 0.06 |
| Cefoxitin | 16 | 8 | 4 |
| Moxalactam | 16 | 1 | 0.12 |
| Aztreonam | >512 | 16 | 0.12 |
| Imipenem | 2 | 0.12 | 0.06 |
| Meropenem | 8 | 0.06 | 0.06 |
Clavulanic acid (CLA) and tazobactam (TZB) were used at concentrations of 2 and 4 μg/ml, respectively.
Genetic analysis.
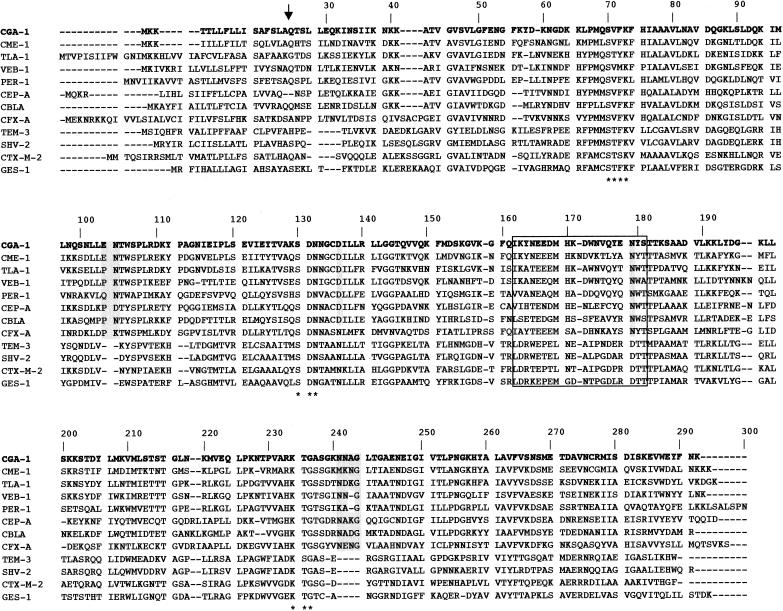
DNA sequence analysis of the 1.4-kb insert of pCGA-1 revealed an open reading frame (ORF) (named blaCGA-1) of 879 bp encoding a 293-amino-acid preprotein (Fig. 1). Typical amino acid residues for Ambler class A β-lactamases were identified in this protein designated CGA-1 (C. gleum class A) (Fig. 1). No sequence typical of a class 1 integron was found within this sequenced insert. No ORF coding for a putative regulatory protein was found in the sequenced DNA upstream of the identified ORF. The overall G+C content of the ORF was 34%, which lies close to the expected range of G+C ratio of the Chryseobacterium sp. genes (36 to 38%) (23).
FIG. 1.
Amino acid sequence comparison of 12 representative class A ESBLs. The amino acid residues highlighted in grey are specific to the β-lactamases of the PER subgroup suggested by Tranier et al. (21), while those of the Ω-loop sequence are boxed. Asterisks indicate the conserved motifs SXXK, SDN, and KTG of Ambler class A β-lactamases (1). Dashes indicate gaps introduced to optimize the alignment. The putative cleavage site for the leader peptide of CGA-1 is indicated by a vertical arrow.
No plasmid was detected in C. gleum CIP 103039. Conjugation experiments failed to transfer any β-lactam resistance marker from C. gleum CIP 103039 to nalidixic acid-resistant E. coli JM109. However, no control was used in the assays for conjugation between C. gleum and E. coli. Using DNA primers located at the end of blaCGA-1, positive PCR results were obtained with either whole-cell DNA of C. gleum CIP 103109 or recombinant plasmid pCGA-1 as a template, thus demonstrating the origin of the cloned gene. Additionally, a PCR fragment internal for blaCGA-1 and used as a probe hybridized with whole-cell DNA at the chromosomal position of migration (data not shown). These results, the G+C content, and the negative results of plasmid detection suggested the likely chromosomal location of blaCGA-1.
Biochemical characterization.
IEF analysis revealed that cultures of E. coli DH10B(pCGA-1) produced a β-lactamase activity with a pI value of 8.9, close to that determined for CME-2 from C. meningosepticum (pI 9.2). A similar β-lactamase activity was detected in a nonpurified β-lactamase extract of a culture of C. gleum CIP 103039. The cleavage site for the leader peptide for the protein sequence deduced from blaCGA-1 was predicted by computer analysis to be between amino acid motifs SLA and QTS (Fig. 1). The mature protein had a relative molecular mass that was determined experimentally to be ca. 31 kDa (data not shown) from a purified β-lactamase of an E. coli(pCGA-1) culture.
A specific activity of 51 μmol · min−1 · mg of protein−1 was determined with 100 μM cephalothin as a substrate for purified β-lactamase CGA-1 extracted from cultures of E. coli DH10B(pCGA-1). The purification coefficient was calculated to be 795-fold, and the purity of the enzyme was estimated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis to be >90%.
The kinetic parameters of the β-lactamase CGA-1 revealed its activity against amino- and carboxy-penicillins, restricted-spectrum cephalosporins, and (at a lower level) extended-spectrum cephalosporins (Table 2). The hydrolytic activities of CGA-1 were lower than those reported for CME-2 (3). CGA-1 had no detectable activity against piperacillin and cephamycins, even though it has been reported for CME-1 and CME-2 (3, 16). CGA-1 activity towards aztreonam could not alone explain the high level of aztreonam resistance observed for C. gleum CIP 103039. The MIC of aztreonam was increased for E. coli DH10B(pCGA-1) compared to that for the E. coli DH10B reference strain, thus showing that CGA-1 activity may in fact exist against this substrate, even though it was not highly correlated with the in vitro activity measurements. Resistance to this monobactam likely resulted also from altered protein binding affinities, low coefficient of outer membrane permeability, or efflux mechanism as reported previously (7). While carbapenem hydrolysis was not detectable with CME-2 (3), CGA-1 possessed a low Km value for imipenem (Table 2). However, the catalytic efficacy (kcat/Km value) of imipenem hydrolysis remained weak. IC50s obtained with benzylpenicillin as the substrate showed that CGA-1 activity was weakly inhibited by clavulanic acid (5 μM) and tazobactam (7 μM). In addition, CGA-1 activities, like CME-2 activities, were weakly inhibited by cefoxitin and imipenem (IC50s of 8 and 50 μM, respectively). According to the kinetic parameters of CGA-1, it could be classified in the same Bush functional group 2be (5) as CME-1 and CME-2.
TABLE 2.
Kinetic parameters of the purified β-lactamase CGA-1a
| β-Lactam | kcat (s−1) | Km (μM) | kcat/Km (mM−1 · s−1) |
|---|---|---|---|
| Benzylpenicillin | 20 | 4 | 5,500 |
| Amoxicillin | 20 | 26 | 800 |
| Ticarcillin | 0.2 | <1 | >0.2 |
| Piperacillin | <0.01 | ND | ND |
| Cefalothin | 30 | 6 | 4,500 |
| Cephaloridine | 20 | 17 | 1,200 |
| Cefuroxime | 70 | 31 | 2,200 |
| Cefoperazone | 10 | 3 | 2,000 |
| Ceftriaxone | 35 | 19 | 1,800 |
| Ceftazidime | 70 | 360 | 200 |
| Cefotaxime | 60 | 52 | 1,100 |
| Cefepime | 10 | 96 | 100 |
| Cefpirome | 10 | 23 | 450 |
| Cefoxitin | <0.002 | ND | ND |
| Moxalactam | <0.002 | ND | ND |
| Imipenem | 0.5 | 3 | 120 |
| Aztreonam | 0.5 | 6 | 60 |
Values are geometric means of three independent measures (standard deviations were within 15%). ND, not detectable activity.
Induction experiments.
Induction studies with cefoxitin as a β-lactam inducer failed to detect the inducibility of CGA-1 expression from cultures of C. gleum CIP 103039. These results were consistent with the absence of any Lys-R-type regulator gene located immediately upstream of blaCGA-1 as reported for blaCME-2 (3).
Amino acid sequence analysis.
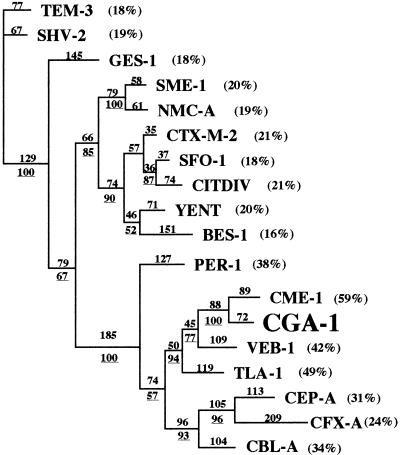
Amino acid sequence alignment with several class A ESBLs showed that CGA-1 shared the highest sequence identity (60%) with CME-1 from C. meningosepticum and was weakly related to other ESBLs (Fig. 2). A dendrogram deduced from an amino acid alignment suggests that CGA-1 belongs to a subgroup of ESBLs consisting of PER-1, CEP-A, CBL-A, CME-1, TLA-1, CFX-A, and VEB-1 (Fig. 2).
FIG. 2.
Dendrogram obtained for 18 Ambler class A ESBLs by the parsimony method (20). Branch lengths are drawn to scale and are proportional to the number of amino acid changes. The percentages at the branching points (underlined) refer to the number of times that a particular node was found in 100 bootstrap replications. The distance along the vertical axis has no significance. The percentages in parentheses are amino acid identities to CGA-1.
CGA-1 possesses several sequence similarities with PER-1 and especially two residues (Asp136 and Asn179) that classified this enzyme as a member of this PER subgroup as recommended by Tranier et al. (21). PER-1 is actually the only enzyme of this subgroup for which the crystal structure has been resolved (21). Three insertions of amino acid residues characteristic of this subgroup of class A β-lactamases are present in the CGA-1 sequence: Glu103a and Asn103b, Pro112a and Ala112b, and four amino acid residues in position Ambler 240 (Fig. 1). In PER-1, the third insertion seems to be implicated in the hydrolysis of bulky expanded-spectrum cephalosporins, increasing the size of the substrate binding pocket. The CGA-1 β-lactamase also possesses an Ala237 residue that increased the kcat/Km value of the Ala237 PER-1 mutant for cephalosporins by 10- to 100-fold (4). Several other amino acids characteristic of the PER subgroup are present in the CGA-1 β-lactamase (19, 21), like Thr104 and His170, and could also be involved in substrate binding.
Conclusion.
After the characterization of CME-1 and CME-2 from C. meningosepticum, this report identifies a second subgroup of class A ESBL that is involved in intrinsic resistance in another Chryseobacterium species. A chromosomally encoded class A ESBL gene as a cause of intrinsic resistance has been reported also in rare gram-negative species, such as in Stenotrophomonas maltophilia (L-2 β-lactamase) and Kluyvera cryocrescens (6, 24). The β-lactamases CME-1, CME-2, and CGA-1 share functional and structural similarities. They may be progenitors of emerging plasmid-mediated enzymes. Indeed, the plasmid-mediated β-lactamase TLA-1, identified from an E. coli clinical isolate (19), possesses 50% amino acid identity with CGA-1 and shares structural and functional similarities. The blaTLA-1 gene has an uncommonly low G+C content (36%) that lies close to that of Chryseobacterium and Flavobacterium β-lactamase genes.
Acknowledgments
This work was financed by a grant from the Ministères de l'Education Nationale et de la Recherche (grant UPRES-JE 2227), Université Paris XI, Paris, France.
We thank L. Poirel for a critical reading of the manuscript and C. Bizet for the gift of the C. gleum reference strain.
REFERENCES
- 1.Ambler, R. P., A. F. Coulson, J. M. Frere, J. M. Ghuysen, B. Joris, M. Forsman, R. C. Levesque, G. Tiraby, and S. G. Waley. 1991. A standard numbering scheme for the class A beta-lactamases. Biochem. J. 276:269-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellais, S., L. Poirel, S. Léotard, T. Naas, and P. Nordmann. 2000. Genetic diversity of carbapenem-hydrolyzing metallo-β-lactamases from Chryseobacterium (Flavobacterium) indologenes. Antimicrob. Agents Chemother. 44:3028-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellais, S., L. Poirel, T. Naas, D. Girlich, and P. Nordmann. 2000. Genetic-biochemical analysis and distribution of the Ambler class A β-lactamase CME-2, responsible for extended-spectrum cephalosporin resistance in Chryseobacterium (Flavobacterium) meningosepticum. Antimicrob. Agents Chemother. 44:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouthors, A. T., N. Dagoneau-Blanchard, T. Naas, P. Nordmann, V. Jarlier, and W. Sougakoff. 1998. Role of residues 104, 164, 166, 238 and 240 in the substrate profile of PER-1 beta-lactamase hydrolysing third-generation cephalosporins. Biochem. J. 330:1443-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bush, K., A. A. Medeiros, and G. A. Jacoby. 1995. A functional classification scheme for beta-lactamases and its correlation with molecular structure. Antimicrob. Agents Chemother. 39:1211-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Decousser, J. W., L. Poirel, and P. Nordmann. 2001. Characterization of a chromosomally encoded extended-spectrum class A β-lactamase from Kluyvera cryocrescens. Antimicrob. Agents Chemother. 45:3595-3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fung-Tomc, J., K. Bush, B. Minassian, B. Kolek, R. Flamm, E. Gradelski, and D. Bonner. 1997. Antimicrobial activity of BMS-180680, a new catechol-containing monobactam. Antimicrob. Agents Chemother. 41:1010-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansen, J. B., and R. H. Olsen. 1978. Isolation of large bacterial plasmids and characterization of the P2 incompatibility group plasmids pMG1 and pMG5. J. Bacteriol. 135:227-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holmes, B., R. J. Owen, A. G. Steigerwalt, and D. J. Brenner. 1984. Flavobacterium gleum, a new species found in human clinical specimens. Int. J. Syst. Bacteriol. 34:21-25. [Google Scholar]
- 10.Jooste, P. J., and C. J. Hugo. 1999. The taxonomy, ecology and cultivation of bacterial genera belonging to the family Flavobacteriaceae. Int. J. Food Microbiol. 53:81-94. [DOI] [PubMed] [Google Scholar]
- 11.Kado, C. I., and S. T. Liu. 1981. Rapid procedure for detection and isolation of large and small plasmids. J. Bacteriol. 145:1365-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Committee for Clinical Laboratory Standards. 1997. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A4. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 13.Nordmann, P., and T. Naas. 1994. Sequence analysis of PER-1 extended-spectrum beta-lactamase from Pseudomonas aeruginosa and comparison with class A beta-lactamases. Antimicrob. Agents Chemother. 38:104-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poirel, L., M. Guibert, D. Girlich, T. Naas, and P. Nordmann. 1999. Cloning, sequence analysis, expression, and distribution of ampC-ampR from Morganella morganii clinical isolates. Antimicrob. Agents Chemother. 43:769-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poirel, L., I. Le Thomas, T. Naas, A. Karim, and P. Nordmann. 2000. Biochemical sequence analyses of GES-1, a novel class A extended-spectrum β-lactamase, and the class 1 integron In52 from Klebsiella pneumoniae. Antimicrob. Agents Chemother. 44:622-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rossolini, G. M., N. Franceschini, L. Lauretti, B. Caravelli, M. L. Riccio, M. Galleni, J.-M. Frère, and G. Amicosante. 1999. Cloning of a Chryseobacterium (Flavobacterium) meningosepticum chromosomal gene (blaACME) encoding an extended-spectrum class A β-lactamase related to the Bacteroides cephalosporinases and the VEB-1 and PER β-lactamases. Antimicrob. Agents Chemother. 43:2193-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 18.Siegman-Igra, Y., D. Schwartz, G. Soferman, and N. Konforti. 1987. Flavobacterium group IIb bacteremia: report of a case and review of Flavobacterium infections. Med. Microbiol. Immunol. 176:103-111. [DOI] [PubMed] [Google Scholar]
- 19.Silva, J., C. Aguilar, G. Ayala, M. A. Estrada, U. Garza-Ramos, R. Lara-Lemus, and L. Ledezma. 2000. TLA-1: a new plasmid-mediated extended-spectrum β-lactamase from Escherichia coli. Antimicrob. Agents Chemother. 44:997-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swofford, D. L. 1989. PAUP (version 3.0): phylogenetic analysis using parsimony. Illinois Natural History Survey, Champaign, Ill.
- 21.Tranier, S., A. T. Bouthors, L. Maveyraud, V. Guillet, W. Sougakoff, and J. P. Samama. 2000. The high resolution crystal structure for class A β-lactamase PER-1 reveals the bases for its increase in breadth of activity. J. Biol. Chem. 275:28075-28082. [DOI] [PubMed] [Google Scholar]
- 22.Ursing, J., and B. Bruun. 1991. Genotypic heterogeneity of Flavobacterium group IIb and Flavobacterium breve demonstrated by DNA-DNA hybridization. APMIS 99:780-786. [PubMed] [Google Scholar]
- 23.Vandamme, P., J.-F. Bernardet, P. Segers, K. Kersters, and B. Holmes. 1994. New perspectives in the classification of the flavobacteria: description of Chryseobacterium gen. nov. Bergeyella gen. nov., and Empedobacter nom. rev. Int. J. Syst. Bacteriol. 44:827-831. [Google Scholar]
- 24.Walsh, T. R., A. P. MacGowan, and P. M. Bennett. 1997. Sequence analysis and enzyme kinetics of the L2 serine beta-lactamase from Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 41:1460-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yabuuchi, E., Y. Hashimoto, T. Ezaki, Y. Ido, and N. Takeuchi. 1990. Genotypic and phenotypic differentiation of Flavobacterium indologenes (Yabuuchi et al. 1983) from Flavobacterium gleum (Holmes et al. 1984). Microbiol. Immunol. 34:73-76. [DOI] [PubMed] [Google Scholar]
- 26.Yabuuchi, E., T. Kaneko, I. Yano, C. W. Moss, and N. Miyoshi. 1983. Sphingobacterium gen. nov., Sphingobacterium spiritivorum comb. nov., Sphingobacterium multivorum comb. nov., Sphingobacterium mizutae sp. nov., and Flavobacterium indologenes sp. nov.: glucose-nonfermenting gram-negative rods in CDC groups IIK-2 and IIb. Int. J. Syst. Bacteriol. 33:580-598. [Google Scholar]