Abstract
T-705 (6-fluoro-3-hydroxy-2-pyrazinecarboxamide) has been found to have potent and selective inhibitory activity against influenza virus. In an in vitro plaque reduction assay, T-705 showed potent inhibitory activity against influenza A, B, and C viruses, with 50% inhibitory concentrations (IC50s) of 0.013 to 0.48 μg/ml, while it showed no cytotoxicity at concentrations up to 1,000 μg/ml in Madin-Darby canine kidney cells. The selectivity index for influenza virus was more than 2,000. It was also active against a neuraminidase inhibitor-resistant virus and some amantadine-resistant viruses. T-705 showed weak activity against non-influenza virus RNA viruses, with the IC50s being higher for non-influenza virus RNA viruses than for influenza virus, and it had no activity against DNA viruses. Orally administered T-705 at 100 mg/kg of body weight/day (four times a day) for 5 days significantly reduced the mean pulmonary virus yields and the rate of mortality in mice infected with influenza virus A/PR/8/34 (3 × 102 PFU). These results suggest that T-705 may be a compound that is useful and highly selective against influenza virus infections and that has a mode of action different from those of commercially available drugs, such as amantadine, rimantadine, and neuraminidase inhibitors.
Influenza is one of the oldest and most common infections causing significant morbidity and mortality. Compared with infections caused by other respiratory viruses like respiratory syncytial virus, rhinoviruses, and enteroviruses, influenza virus infections can cause more severe complications such as pneumonia and ischemic heart disease and can increase the rates of hospitalization and mortality, particularly in young children and elderly people (6, 20). To date, the M2 ion channel inhibitors, amantadine and rimantadine, have been used for treatment of influenza virus infections worldwide. Amantadine and rimantadine have similar levels of activity, but rimantadine is associated with significantly fewer adverse reactions (2). These drugs are known to reduce the severity and duration of illness when they are taken at the time of onset of symptoms. However, their efficacies against influenza A virus are restricted, and the occurrence of resistant influenza A viruses is inevitable (11, 12). Two new neuraminidase inhibitors, zanamivir and oseltamivir (GS 4104; the prodrug of GS 4071), have recently been used for the treatment of influenza virus infections in the United States and some European countries. Their therapeutic efficacies have been demonstrated in clinical studies (9, 19, 26).
The guanosine analogue ribavirin has been reported to be active against various viruses including influenza A and B viruses by inhibiting RNA synthesis (4, 7, 24). In the United States, aerosolized ribavirin can be used only in the treatment of selected hospitalized infants and young children with severe lower respiratory tract infections due to respiratory syncytial virus but not against influenza virus infections (3, 27).
We have screened for anti-influenza virus compounds and found an orally active anti-influenza agent, T-705. T-705, a pyrazine derivative, showed strong anti-influenza virus activities in some in vitro and in vivo assays. In this study T-705 has been shown to have a broad spectrum of anti-influenza virus activity with no cytotoxicity and strong therapeutic efficacy in a lethal infection model in mice.
MATERIALS AND METHODS
Reagents and compounds.
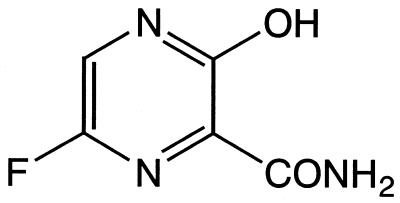
T-705 (6-fluoro-3-hydroxy-2-pyrazinecarboxamide) (Fig. 1) and GS 4071 [(3R,4R,5S)-4-acetamido-5-amino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylic acid], the active form of oseltamivir, were synthesized at Toyama Chemical Co., Ltd. Amantadine (1-aminoadamantane hydrochloride) and ribavirin (1-d-ribofuranosyl-1,2,4-triazole-3-carboxamide) were purchased from Sigma Chemical Co. (St. Louis, Mo.). All compounds were dissolved in Eagle's modification of minimum essential medium (EMEM; Sigma) and were then further diluted in each test medium.
FIG. 1.
Structure of T-705.
Cells.
MDCK (Madin-Darby canine kidney) cells, A549 (human lung carcinoma) cells, and HEp-2 (human laryngeal carcinoma) cells were purchased from the American Type Culture Collection (ATCC; Manassas, Va.). HeLa (human cervical carcinoma) cells, Vero (embryonic African green monkey kidney) cells, and HEL (human embryonic lung) cells were maintained in our laboratory. All tissue culture cells were routinely grown in EMEM supplemented with 10% fetal calf serum (FCS; Iwaki, Tokyo, Japan) and 60 μg of kanamycin per ml.
Viruses.
Influenza viruses A/FM/1/47 (H1N1), A/NWS/33 (H1N1), A/Japan/305/57 (H2N2), and A/Port Chalmers/1/73 (H3N2) were purchased from ATCC. Influenza virus A/PR/8/34 (H1N1) and clinically isolated influenza viruses A/Yamagata/120/86 (H1N1), A/Suita/1/89 (H1N1), A/Kaizuka/2/65 (H2N2), A/Okuda/57 (H2N2), A/Takathuki/4/65 (H3N2), A/Aichi/2/68 (H3N2), A/Ibaraki/1/90 (H3N2), A/Kitakyushu/159/93 (H3N2), B/Nagasaki/1/87, B/Guandong/5/94, and B/Mie/1/93 were gifts from Y. Okuno (Osaka Prefectural Institute of Public Health). Influenza virus C/Taylor/1233/47, clinical isolates of influenza C/Yamagata/3/96, and JJ/50 viruses were gifts from K. Nakamura (Yamagata University). All influenza viruses were propagated in MDCK cells.
Herpes simplex virus type 1 (HSV-1) strain 7401H and the poliovirus I strain Sabin, were maintained in our laboratory and were propagated in Vero cells. Human cytomegalovirus (HCMV) strain Towne was also maintained in our laboratory and was propagated in HEL cells. A human adenovirus type 3 strain, rhinovirus type 2 strain HGP, and respiratory syncytial virus (RSV) strain A-2 were purchased from ATCC and were propagated in A549 cells, HeLa cells, and HEp-2 cells, respectively. A GS 4071-resistant influenza virus was isolated from influenza virus A/PR/8/34 that had been serially passaged six times in MDCK cells in the presence of GS 4071.
Mice.
Specific-pathogen-free male BALB/c mice (age, 4 weeks) were obtained from Japan SLC Inc. (Shizuoka, Japan). They were quarantined for 1 day prior to infection and were maintained on rodent diet from CLEA Japan Inc. (Tokyo, Japan) and distilled water from Otsuka Pharmaceutical Co., Ltd. (Tokyo, Japan).
Anti-influenza virus activity.
Anti-influenza virus activity was evaluated by plaque reduction assays. Confluent monolayers of MDCK cells in six-well tissue culture plates were inoculated with 70 PFU of virus per well. After 60 min, the inoculum was removed and the test medium containing the desired concentration of compounds was added. MDCK cells inoculated with influenza A and B viruses were incubated under 100% humidity and 5% CO2 in a 0.5% agarose medium containing 0.001% DEAE-dextran and 2 μg of trypsin per ml for 2 days at 35°C. The cells inoculated with influenza C virus were incubated under 100% humidity and 5% CO2 in a 0.8% agarose medium with 1 μg of folic acid per ml, 1 μg of biotin per ml, 0.1% glucose, 1% albumin, 0.01% DEAE-dextran, and 5 μg of trypsin per ml for 6 days at 33°C. Then, the test plates were fixed with 3% formaldehyde solution and the overlay was removed. The cells were stained with 0.005% amido black solution and the plaque numbers were counted. The 50% inhibitory concentrations (IC50s) were determined and were the concentrations required to reduce the number of plaques to 50% of the number in wells containing no compounds.
Activity against GS 4071-resistant virus was evaluated by the yield reduction assay. Confluent monolayers of MDCK cells in 24-well tissue culture plates were inoculated with 200 PFU of virus per well (multiplicity of infection, 0.001). After 60 min, the inoculum was removed and the cells were overlaid and incubated with the test medium containing the desired concentration of compounds, 1% albumin, 3% vitamin solution (GIBCO), and 2 μg of trypsin per ml for 24 h. Then, the IC90s were determined and were the concentrations required to reduce the virus yield to 10% of that in wells containing no compounds.
Activities of T-705 against non-influenza viruses.
The activities of T-705 against HSV-1, poliovirus (16), HCMV (30), adenovirus (13), rhinovirus (5), and RSV (8) were evaluated by the plaque reduction assay by methods reported elsewhere. A total of 50 to 100 PFU of each virus was allowed to adsorb to the appropriate confluent cell lines, as stated above, for 60 min, followed by washing of each virus with a medium without serum. Then, the test medium containing the desired concentration of compounds was added. After appropriate periods of incubation, the culture was fixed and stained and then the plaque numbers were counted. The IC50s were determined as described above.
Cytotoxicity.
The cytotoxicity of T-705 was evaluated by an assay with 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2 H-tetrazolium hydroxide (XTT) (22). XTT is converted to aqueous formazan by an enzyme in MDCK cells, Vero cells, HEL cells, A549 cells, HeLa cells, and HEp-2 cells. The compounds were diluted to the appropriate concentrations (volume, 100 μl) with test medium (EMEM containing 10% FCS) in 96-well culture plates in which each well contained a concentration of 2 × 103 cells/100 μl. The test plates were incubated for 3 days at 37°C in 100% humidity and 5% CO2. After 3 days, 50 μl of the XTT reagent (1 mg/ml in FCS-free EMEM containing 5 mM phenazine methosulfate) was added, and the reaction product was assayed by measurement of the absorbance at 450 nm with a microplate reader. Cytotoxicity was expressed as the 50% cell-inhibitory concentration (CC50).
Therapeutic efficacy in mice.
Mice (weight, 17 to 19 g) were anesthetized with methyl ether and exposed to 20 μl of mouse-adapted influenza virus A/PR/8/34 (3 × 102 PFU/mouse) by intranasal instillation. The mice were divided into four groups; and T-705 at a dose of 50, 100, or 200 mg/kg of body weight/day or a placebo was orally administered to the mice four times daily (q.i.d.; at 6-h intervals) for 5 days beginning 1 h after infection. The placebo controls were treated with 0.5% methylcellulose solution. In the survival rate study (n = 14), the mice were observed for mortality daily for 21 days after infection. In the lung virus yield study (n = 7 to 10), the mice were killed at 6 days postinfection while they were under ether anesthesia, and the lung virus yields were determined by plaque assays. The log rank test was used to evaluate differences in the survival rates of the mice. Differences in lung virus yields compared with the control value were evaluated by the nonparametric Dunnett's test. In cases in which lung virus yields were less than the limit of detection (2 × 102 PFU/lung), the yields were approximated to 100 PFU/lung. Procedures involving animals and their care were conducted in conformance with the experimentation guidelines of the Toyama Medical and Pharmaceutical University, which are in compliance with international law and policies.
RESULTS
Anti-influenza virus activity.
The results of in vitro plaque reduction assays with T-705, GS 4071, amantadine, and ribavirin are shown in Table 1. T-705 inhibited the formation of plaques by all laboratory-adapted and clinical isolates of influenza A, B, and C viruses. The IC50s ranged from 0.013 to 0.48 μg/ml for the influenza A viruses, from 0.039 to 0.089 μg/ml for the influenza B viruses, and from 0.030 to 0.057 μg/ml for the influenza C viruses.
TABLE 1.
Anti-influenza virus activities of T-705, GS 4071, amantadine, and ribavirin
| Virus type and strain | IC50 (μg/ml)a
|
|||
|---|---|---|---|---|
| T-705 | GS 4071 | Amantadine | Ribavirin | |
| A (H1N1) | ||||
| PR/8/34 | 0.16 | 0.0039 | >50 | 7.7 |
| FM/1/47 | 0.20 | 0.0056 | 0.30 | 1.6 |
| NWS/33 | 0.091 | 0.0020 | >50 | 12 |
| Yamagata/120/86 | 0.12 | 0.0096 | 1.6 | 4.1 |
| Suita/1/89 | 0.029 | 0.0025 | >50 | 8.7 |
| A (H2N2) | ||||
| Kaizuka/2/65 | 0.029 | 0.68 | 0.15 | 8.3 |
| Okuda/57 | 0.013 | 0.00044 | 0.25 | 4.9 |
| Japan/305/57 | 0.30 | 0.00039 | 0.062 | 13.8 |
| Takathuki/4/65 | 0.048 | 0.00017 | 0.19 | Not tested |
| A (H3N2) | ||||
| Port Chalmers/1/73 | 0.46 | 0.00060 | Not tested | 5.8 |
| Aichi/2/68 | 0.078 | 0.0030 | 0.63 | 4.3 |
| Ibaraki/1/90 | 0.30 | 0.00049 | 0.47 | 4.8 |
| Kitakyushu/159/93 | 0.48 | 0.00056 | >50 | 20 |
| B | ||||
| Nagasaki/1/87 | 0.089 | 0.0063 | >50 | 19 |
| Guandong/5/94 | 0.053 | 0.031 | >50 | 1.2 |
| Mie/1/93 | 0.039 | 0.015 | >50 | 4.6 |
| C | ||||
| Taylor/1233/47 | 0.044 | >100 | Not tested | Not tested |
| Yamagata/3/96 | 0.057 | >100 | Not tested | Not tested |
| JJ/50 | 0.030 | >100 | Not tested | Not tested |
| GS 4071-resistant virus A/PR/8/34b | 0.095c | 25c | Not tested | Not tested |
The Values are averages of results from two or three independent experiments.
Isolated by passage in GS 4071 six times.
The values are IC90s (the concentrations required to reduce the viral yield by 1 log10).
GS 4071 showed inhibitory activity against influenza A viruses (IC50s, 0.00017 to 0.68 μg/ml) and influenza B viruses (IC50s, 0.0063 to 0.031 μg/ml) but lacked inhibitory activity against influenza C viruses (IC50s, >100 μg/ml). A clinical isolate of influenza virus, A/Kaizuka/2/65 (H2N2), showed a lower level of susceptibility to GS 4071 (IC50, 0.68 μg/ml). After six passages in the presence of GS 4071, influenza virus A/PR/8/34 exhibited a decrease in susceptibility to GS 4071 (IC90, 25 μg/ml). T-705 was as active against this GS 4071-resistant virus as it was against the parent virus, and the T-705 IC50s were 0.095 and 0.16 μg/ml for the former and the latter viruses, respectively.
Amantadine showed inhibitory activity against influenza A viruses, with IC50s ranging from 0.062 to >50 μg/ml. The two laboratory-adapted influenza A viruses (A/PR/8/34 [H1N1] and NWS/33 [H1N1]) as well as the two clinical isolates of influenza A viruses (Suita/1/89 [H1N1] and Kitakyushu/159/93 [H3N2]) exhibited decreased susceptibilities to amantadine. T-705 showed inhibitory activities against these viruses, with IC50s of 0.16, 0.091, 0.029, and 0.48 μg/ml, respectively.
When the activity of T-705 was compared to that of ribavirin against the same subtypes of influenza A virus (H1N1, H2N2, H3N2) and influenza B virus, the IC50s of T-705 were consistently found to be lower than those of ribavirin.
Activity of T-705 against non-influenza viruses.
T-705 did not inhibit the formation of plaques by HSV-1, HCMV, or adenovirus, with IC50s being >100 μg/ml. Poliovirus, rhinovirus, and RSV were susceptible to T-705, with IC50s of 4.8, 23, and 41 μg/ml, respectively (Table 2).
TABLE 2.
Spectrum of activity of T-705 against non-influenza virus
| Virus and cell | T-705 IC50 (μg/ml)a | |||
|---|---|---|---|---|
| Type | Family | Species | Host cell | |
| DNA | Herpesviridae | HSV-1 | Vero | >100 |
| Herpesviridae | HCMV | HEL | >100 | |
| Adenoviridae | Adenovirus | A549 | >100 | |
| RNA | Picornaviridiae | Poliovirus | Vero | 4.8 |
| Picornaviridae | Rhinovirus | HeLa | 23 | |
| Paramyxoviridae | RSV | HEp-2 | 41 | |
The values are averages of results from two independent experiments.
Cytotoxicities of T-705 and reference compounds.
The cytotoxicities of T-705 for mammalian cell lines (MDCK cells, Vero cells, HEL cells, A549 cells, HeLa cells, and HEp-2 cells) were compared with those of GS 4071, amantadine, and ribavirin. T-705 and GS 4071 showed no cytotoxicity against these cell lines at concentrations up to 1,000 μg/ml. However, amantadine and ribavirin showed cytotoxicity against these cell lines, with CC50s of 18 to 160 and 8 to 75 μg/ml, respectively (Table 3).
TABLE 3.
Cytotoxicities of T-705 and reference compounds
| Cell line | CC50 (μg/ml)a
|
|||
|---|---|---|---|---|
| T-705 | GS 4071 | Amantadine | Ribavirin | |
| MDCK | >1,000 | >1,000 | 160 | 23 |
| Vero | >1,000 | >1,000 | 140 | 59 |
| HEL | >1,000 | >1,000 | 18 | 19 |
| A549 | >1,000 | >1,000 | 81 | 75 |
| HeLa | >1,000 | >1,000 | 89 | 11 |
| HEp-2 | >1,000 | >1,000 | 91 | 7.8 |
The values are averages of results from two independent experiments.
Therapeutic efficacy of T-705 in mice.
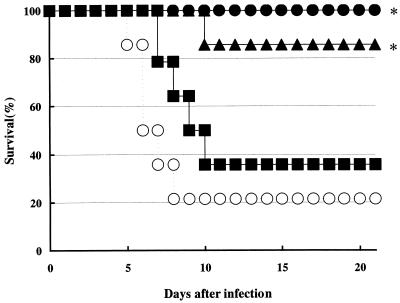
The therapeutic efficacy of T-705 was evaluated on the basis of the survival rate at 21 days postinfection and the lung virus yield at 6 days postinfection in influenza virus A/PR/8/34-infected mice. Orally administered T-705 prevented influenza virus-induced deaths in a dose-dependent manner. T-705 at doses of 200 mg/kg/day for 5 days protected the mice from death from influenza virus infection (P < 0.0125). It also had a significant therapeutic effect in terms of the survival rates (85.7%; P < 0.0125) for those treated with 100 mg/kg/day (q.i.d.). For the group treated with T-705 at doses of 50 mg/kg/day and methylcellulose solution-treated control mice, the survival rates were 35.7 and 21.4%, respectively (Fig. 2).
FIG. 2.
Effect of oral administration of T-705 on prevention of death in influenza virus-infected mice. Mice were infected with influenza virus A/PR/8/34 at 3 × 102 PFU/mouse as described in Materials and Methods. Mice were treated q.i.d. with oral doses of T-705 at 50 (▪), 100 (▴), or 200 (•) mg/kg/day or with methylcellulose solution as a control (○) for 5 days beginning 1 h after infection. The results presented here were obtained from a single representative experiment. ∗, P < 0.0125 compared to the results for 0.5% methylcellulose solution-treated controls (log rank test).
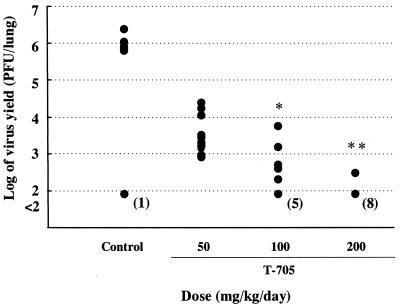
Reductions in lung virus yields in T-705-treated mice were also observed to occur in a dose-dependent manner. In the groups treated with T-705 at 50, 100, and 200 mg/kg/day, the mean yields were reduced to 3.50, 2.45 (P < 0.05), and 2.10 (P < 0.01) log10 PFU/lung, respectively, whereas the yields in control mice were 5.47 log10 PFU/lung. The lung virus yields were less than the limit of detection in 50% of the mice treated with 100 mg/kg and in 80% of the mice treated with 200 mg/kg (Fig. 3).
FIG. 3.
Effect of oral administration of T-705 on lung virus yield in influenza virus-infected mice. Mice were infected with influenza virus A/PR/8/34 at 3 × 102 PFU/mouse, and lung virus yields were determined as described in Materials and Methods. Mice were treated q.i.d. with oral doses of T-705 at 50, 100, or 200 mg/kg/day or with methylcellulose solution as a control for 5 days beginning 1 h after infection. The results presented here were obtained from a single representative experiment. The numbers in parentheses represent the number of mice whose lung virus titers were less than the limit of detection (2 × 102 PFU/lung). ∗, P < 0.05 compared to the results for 0.5% methylcellulose solution-treated controls (nonparametric Dunnett's test); ∗∗, P < 0.01 compared to the results for 0.5% methylcellulose solution-treated controls (nonparametric Dunnett's test).
DISCUSSION
We have been exploring orally administered agents active against influenza viruses and have found a pyrazine derivative, T-705, which has a simple structure and which displays potent activity against influenza viruses. This report describes cell culture studies and the antiviral activity of T-705 in appropriate animal infection models. Interestingly, in the in vitro assays, T-705 showed potent inhibitory activities against all types of influenza A, B, and C viruses. Amantadine and rimantadine have activities only against influenza A viruses. These drugs inhibit viral fusion and uncoating by binding to the viral M2 protein (21, 28). As influenza B and C viruses do not encode an M2 integral membrane protein, amantadine exhibits activity only against influenza A viruses (10). Zanamivir and oseltamivir have specific inhibitory activities against viral neuraminidase, one of the major surface glycoproteins, which is expressed by both influenza A and B viruses (14, 15). These drugs are active against influenza A and B viruses but not against influenza C viruses (18). The observation that T-705 inhibited any type of influenza virus suggests that the mode of action of T-705 may be different from those of amantadine, rimantadine, and neuraminidase inhibitors.
It has been reported that ribavirin has activity against both DNA and RNA viruses (17, 23, 29). In our study, ribavirin inhibited the replication of some RNA viruses, poliovirus, rhinovirus, and RSV, with IC50s of 240, 33, and 5.1 μg/ml, respectively (unpublished data). T-705 did not inhibit the replication of DNA viruses and showed weak activity against non-influenza virus RNA viruses, such as poliovirus, rhinovirus, and RSV, with the IC50s for these RNA viruses being higher than those for the influenza viruses. While T-705 at concentrations up to 1,000 μg/ml showed no cytotoxicity for the mammalian cells tested, ribavirin exhibited cytotoxicity against these cells. The selectivity index (the ratio of the CC50 for growing cells to the IC50 for the virus) was greater than 2,000 for T-705, whereas it was less than 63 for ribavirin. From these observations and the differences in antiviral spectra and selectivities, it has been suggested that T-705 may have a mode of action different from that of ribavirin. The monophosphate is considered one of the active forms of ribavirin, and it inhibits IMP dehydrogenase and brings about a reduction in the intracellular concentration of GTP (24). Recently, it has been demonstrated that ribavirin triphosphate is incorporated by the poliovirus RNA polymerase 3Dpol and that the incorporated ribavirin is mutagenic. The antiviral activity of ribavirin correlated directly with its mutagenic activity (1). Because of these reports, detailed studies on the mechanism of action of T-705 are in progress.
T-705 displayed potent activity against laboratory-adapted and clinically isolated amantadine-resistant and GS 4071-resistant influenza A viruses. It has been reported that rimantadine-resistant strains of influenza virus are frequently recovered from rimantadine-treated children and adults by day 5 of treatment and may subsequently be transmitted to contacts, so the prophylactic effectiveness is limited under conditions of close contact, like in a family setting (11, 12). While a GS 4071-resistant virus was selected by 12 passages in vitro in the presence of the inhibitor and it exhibited >3,000 times less susceptibility, the infectivity of the virus was reduced in mice (25). It is not yet obvious that the resistance of viruses to neuraminidase inhibitors would be problematic during clinical use, but T-705 may retain its activity against these resistant viruses. After eight passages of influenza virusA/PR/8/34 in MDCK cells in the presence of T-705, no obvious change in susceptibility was seen (data not shown). Future studies will need to confirm the low propensity for the development of resistance to T-705.
In this study, orally administered T-705 demonstrated protective activity against lethal influenza virus infections in mice, and lung virus yields were less than the limit of detection in some animals when T-705 was used at a dose of 100 mg/kg/day or higher.
In conclusion, T-705 is considered a good candidate as an anti-influenza virus agent because of its selective inhibitory activity against influenza viruses.
Acknowledgments
We thank Yoshinobu Okuno (Osaka Prefectural Institute of Public Health) and Kiyohito Nakamura (Yamagata University) for providing the influenza viruses. We also thank Masahiko Kurokawa, Seiji Kageyama, and a member of the Department of Virology of Toyama Medical and Pharmaceutical University for discussions and encouragement during the course of this work.
REFERENCES
- 1.Crotty, S., D. Maag, J. J. Arnold, W. Zhong, J. Y. N. Lau, Z. Hong, R. Andino, and C. E. Cameron. 2000. The broad-spectrum antiviral ribonucleoside ribavirin is an RNA virus mutagen. Nat. Med. 6:1375-1379. [DOI] [PubMed] [Google Scholar]
- 2.Dolin, R., C. R. Richard, M. H. Paul, M. Raina, N. L. Pamela, and W. J. Joan. 1982. A controlled trial of amantadine and rimantadine in the prophylaxis of influenza A infection. N. Engl. J. Med. 307:580-584. [DOI] [PubMed] [Google Scholar]
- 3.Eggleston, M. 1987. Clinical review of ribavirin. Infect. Control 8:215-218. [DOI] [PubMed] [Google Scholar]
- 4.Eriksson, B., E. Helgstrand, N. G. Johansson, A. Larsson, A. Misiorny, J. O. Noren, L. Philipson, K. Stenberg, G. Stening, S. Stridh, and B. Öberg. 1977. Inhibition of influenza virus ribonucleic acid polymerase by ribavirin triphosphate. Antimicrob. Agents Chemother. 11:946-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fiala, M., and G. Kenny. 1966. Enhancement of rhinovirus plaque formation in human heteroploid cell cultures by magnesium and calcium. J. Bacteriol. 92:1710-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glezen, W. P. 1982. Serious morbidity and mortality associated with influenza epidemics. Epidemiol. Rev. 4:25-44. [DOI] [PubMed] [Google Scholar]
- 7.Goswami, B. B., E. Borek, O. K. Sharma, J. Fujitaki, and R. A. Smith. 1979. The broad spectrum antiviral agent ribavirin inhibits capping of mRNA. Biochem. Biophys. Res. Commun. 89:830-836. [DOI] [PubMed] [Google Scholar]
- 8.Graham, B. S., M. D. Perkins, P. F. Wright, and D. T. Karzon. 1988. Primary respiratory syncytial virus infection in mice. J. Med. Virol. 26:153-162. [DOI] [PubMed] [Google Scholar]
- 9.Hayden, F. G., A. D. M. E. Osterhaus, J. J. Treanor, D. M. Fleming, F. Y. Aoki, K. G. Nicholson, A. M. Bohnen, H. M. Hirst, O. Keene, and K. Wightman. 1997. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenzavirus infections. N. Engl. J. Med. [DOI] [PubMed]
- 10.Hayden, F. G., K. M. Cote, and R. G. Douglas. 1980. Plaque inhibition assay for drug susceptibility testing of influenza viruses. Antimicrob. Agents Chemother. 17:865-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayden, F. G., R. B. Robert, R. D. Clover, A. J. Hay, M. G. Oakes, and W. Soo. 1989. Emergence and apparent transmission of rimantadine-resistant influenza A virus in families. N. Engl. J. Med. 321:1696-1702. [DOI] [PubMed] [Google Scholar]
- 12.Hayden, F. G., S. J. Sperber, R. B. Belshe, R. D. Clover, A. J. Hay, and S. Pyke. 1991. Recovery of drug-resistant influenza A virus during therapeutic use of rimantadine. Antimicrob. Agents Chemother. 35:1741-1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hui, M. B. V., E. J. Lien, and M. D. Trousdale. 1994. Inhibition of human adenoviruses by 1-(2′-hydroxy-5′-methoxybenzylidene)amino-3-hydroxyguanidine tosylate. Antivir. Res. 24:261-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Itzstein, M., W. Y. Wu, G. B. Kok, M. S. Pegg, J. C. Dyason, B. Jin, T. V. Phan, M. L. Smythe, H. F. White, S. W. Oliver, P. M. Colman, J. N. Varghese, D. M. Ryan, J. M. Woods, R. C. Bethell, V. J. Hotham, J. M. Cameron, and C. R. Penn. 1993. Rational design of potent sialidase-based inhibitors of influenza virus replication. Nature 363:418-423. [DOI] [PubMed] [Google Scholar]
- 15.Kim, C. U., W. Lew, M. A. Williams, H. Wu, L. Zhang, X. Chen, P. A. Escarpe, D. B. Mendel, W. G. Laver, and R. C. Stevens. 1998. Structure-activity relationship studies of novel carbocyclic influenza neuraminidase inhibitors. J. Med. Chem. 41:2451-2460. [DOI] [PubMed] [Google Scholar]
- 16.Kurokawa, M., H. Ochiai, K Nagasaka, M. Neki, H. Xu, S. Kadota, S. Sutardjo, T Matsumoto, T Namba, and K. Shiraki. 1993. Antiviral traditional medicines against herpes simplex virus (HSV-1), poliovirus and measles virus in vitro and their therapeutic efficacies for HSV-1 infection in mice. Antivir. Res. 22:175-188. [DOI] [PubMed] [Google Scholar]
- 17.Markland, W., T. J. Mcquaid, J. Jain, and A. D. Kwong. 2000. Broad-spectrum antiviral activity of the IMP dehydrogenase inhibitor VX-497: a comparison with ribavirin and demonstration of antiviral additivity with alpha interferon. Antimicrob. Agents Chemother. 44:859-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mendel, D. B., C. Y. Tai, P. A. Escarpe, W. Li, R. W. Sidwell, J. H. Huffman, C. Sweet, K. J. Jakeman, J. Merson, S. A. Lacy, W. Lew, M. A. Williams, L. Zhang, M. S. Chen, N. Bischofberger, and C. U. Kim. 1998. Oral administration of a prodrug of the influenza virus neuraminidase inhibitor GS 4071 protects mice and ferrets against influenza infection. Antimicrob. Agents Chemother. 42:640-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nicholson, K. G., F. Y. Aoki, A. D. M. E. Osterhaus, S. Trottier, O. Carewicz, C. H. Mercier, A. Rode, N. Kinnersley, and P. Ward. 2000. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Lancet 355:1845-1850. [DOI] [PubMed] [Google Scholar]
- 20.Perrotta, D. M., M. Decker, and W. P. Glezen. 1985. Acute respiratory disease hospitalizations as a measure of impact of epidemic influenza. Am. J. Epidemiol. 122:468-476. [DOI] [PubMed] [Google Scholar]
- 21.Pionto, L. H., L. J. Holsinger, and R. A. Lamp. 1992. Influenza virus M2 protein has ion channel activity. Cell 69:517-528. [DOI] [PubMed] [Google Scholar]
- 22.Scudiero, D. A., R. H. Shoemaker, K. D. Paull, A. Monks, S. Tierney, T. H. Nofziger, M. J.Currens, D. Seniff, and M. R. Boyd. 1988. Evaluation of a tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 48:4827-4833. [PubMed] [Google Scholar]
- 23.Sidwell, R. W., J. H. Huffman, G. P. Khare, L. B. Allen, J. T. Witkowski, and R. K. Robins. 1972. Broad-spectrum antiviral activity of virazole: 1-β-d-ribofuranosyl-1,2,4-triazole-3-carboxamide. Science 177:705-706. [DOI] [PubMed] [Google Scholar]
- 24.Streeter, D. G., J. T. Witkowski, G. P. Khare, R. W. Sidwell, R. J. Bauer, R. K. Robins, and L. N. Simon. 1973. Mechanism of action of 1-β-d-ribofuranosyl-1,2,4-triazole-3-carboxamide (virazole), a new broad-spectrum antiviral agent. Proc. Natl. Acad. Sci. USA 70:1174-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tai, C. Y., P. A. Escarpe, R. W. Sidwell, M. A. Williams, W. Lew, H. Wu, C. U. Kim, and D. B. Mendel. 1998. Characterization of human influenza virus variants selected in vitro in the presence of the neuraminidase inhibitor GS 4071. Antimicrob. Agents Chemother. 42:3234-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Treanor, J. J., F. G. Hayden, P. S. Vrooman, R. Barbarash, R. Bettis, D. Riff, S. Singh, N. Kinnersley, P. Ward, and R. G. Mills. 2000. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza. JAMA 283:1016-1024. [DOI] [PubMed] [Google Scholar]
- 27.U.S. Food and Drug Administration.1986. Ribavirin aerosol approved for severe cases of RSV in infants and young children. FDA Drug Bull. 16:7.. [PubMed] [Google Scholar]
- 28.Wang, C., K. Takeuchi, L. H. Pinto, and R. A. Lamb. 1993. Ion channel activity of influenza A virus M2 protein: characterization of the amantadine block. J. Virol. 67:5585-5594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Witkowski, J. T., R. K. Robins, R. W. Sidwell, and L. N. Simon. 1972. Design, synthesis, and broad spectrum antiviral activity of 1-β-d-ribofuranosyl-1,2,4-triazole-3-carboxamide and related nucleosides. J. Med. Chem. 15:1150-1154. [DOI] [PubMed] [Google Scholar]
- 30.Yukawa, T. A., M. Kurokawa, H. Sato, Y. Yoshida, S. Kageyama, T. Hasegawa, T. Namba, M. Imakita, T. Hozumi, and K. Shiraki. 1996. Prophylactic treatment of cytomegalovirus infection with traditional herbs. Antivir. Res. 32:63-70. [DOI] [PubMed] [Google Scholar]