Abstract
A 14-member macrolide was found to inhibit interleukin-8 (IL-8) synthesis in lipopolysaccharide-stimulated neutrophils but did not accelerate apoptosis in activated neutrophils. These data suggest that 14-member macrolides achieve clinical efficacy for chronic airway diseases partly by suppressing IL-8 production by activated neutrophils, but not by enhancing apoptosis in these cells.
Inflammation in the airways of patients with chronic airway diseases (CAD), such as diffuse panbronchiolitis (DPB), is characterized by dense neutrophil infiltration (6, 7, 17). Our data and those of others have confirmed that a perpetual cycle involving interleukin-8 (IL-8) production and neutrophil accumulation exists in the airway and that bronchial epithelial cells and neutrophils serve as important sources of this chemokine (17, 21). Furthermore, it has been shown that long-term therapy with low doses of erythromycin (EM) or clarithromycin (CAM) is effective for patients with CAD including DPB (7-9, 17, 20, 21). The mechanism of action of the 14-member macrolides in the case of CAD appears to involve their antiinflammatory effects (1, 7, 17, 20).
We have recently demonstrated that neutrophils that have accumulated in the airways of patients with DPB produce IL-8 and undergo apoptosis (H. Yoshimine, K. Oishi, Y. Tsuchihashi, K. Matsushima, and T. Nagatake, abstract from the 2000 International Conference of the American Thoracic Society, Am. J. Respir. Crit. Care Med. 161:A338, 2000), although apoptosis is delayed in neutrophils that have migrated into the airways (11, 25). Apoptotic neutrophils are ingested by macrophages without the release of inflammatory mediators via surface recognition mechanisms (4, 12, 13, 19), while necrotic cells liberate neutrophil serine proteinases, such as neutrophil elastase, and exacerbate the inflammatory response (16, 17). This process is generally accepted as a central mechanism by which inflammatory lung diseases are resolved (5, 13). It would, therefore, be of interest to determine whether macrolides including EM affect apoptosis and the production of IL-8 by activated neutrophils. We report here an evaluation of the effects of 14-member macrolides and other antibiotics on both IL-8 production and apoptosis of human neutrophils with or without lipopolysaccharide (LPS) stimulation.
Dexamethasone 21-acetate (DEX; Sigma Chemical Co., St. Louis, Mo.), MG-132 (BIOMOL Research Laboratories, Inc., Plymouth Meeting, Pa.), erythromycin A (EMA), CAM, josamycin (JM), ampicillin (AMP), and cefaclor (CCL) were dissolved in dimethyl sulfoxide (DMSO) and subsequently diluted in Iscove's modified Dulbecco's medium (IMDM). Neutrophils were purified from the peripheral blood of a healthy volunteer as previously described (17) and suspended at a concentration of 5 × 106 cells/ml in IMDM with 10% autologous sera in 96-well flat-bottom flexible plates (Becton Dickinson, Oxnard, United Kingdom). Neutrophils were incubated with either DEX, MG-132, or an antibiotic at the indicated concentrations for 1 h, after which LPS from Pseudomonas aeruginosa serotype 10 (Sigma) was added to the wells, and cells were cultured in the presence of 10% heat-inactivated autologous sera at 37°C under 5% CO2. After incubation, culture supernatants were stored at −80°C until use. Neutrophils were collected from the flexible plate, centrifuged, stained, and counted in order to determine the percentage of cells with a highly distinctive apoptotic morphology (10, 24, 25). To measure cell membrane changes associated with apoptosis, collected neutrophils were labeled with annexin V-fluorescein isothiocyanate (Beckman Coulter Co., Marseille, France), which labels membrane phosphatidylserine residues, and were analyzed by flow cytometry (24). IL-8 protein levels in culture supernatants were determined as previously described (17). Neutrophils were incubated with each reagent or with an antibiotic at 5 × 106 cells per ml in 96-well culture plates for 48 h, as described previously, to determine cytotoxicity (23). For Northern blot analysis, neutrophils were incubated with either EM, CAM, or DEX at a concentration of 5 × 106 cells/ml in the presence of 10% heat-inactivated autologous sera for 1 h, followed by stimulation with LPS at 1 μg/ml for 30 min in flexible plates. After neutrophils were collected, total RNA from 3 × 107 cells was extracted and levels of IL-8 and control glyceraldehyde-3-phosphate dehydrogenase (G3PDH) mRNA transcripts were evaluated as described previously (26). The percentage of apoptotic cells or the IL-8 levels were compared by one-way analysis of variance and multiple-comparison methods using the Bonferroni-Dunn test. Data were considered statistically significant if P values were less than 0.05.
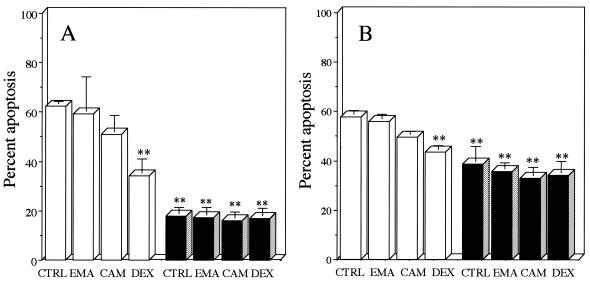
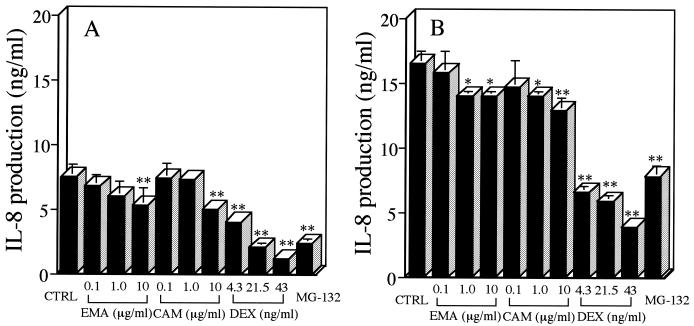
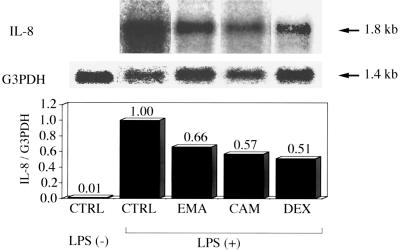
DEX significantly suppressed the percentage of spontaneous neutrophil apoptosis, as estimated by morphology (Fig. 1A) and annexin V binding (Fig. 1B), at 24 h postincubation compared with that for untreated controls. This inhibitory effect of DEX on spontaneous apoptosis has been demonstrated previously by Cox and Austin (3). Others have shown that stimulation with LPS (1 μg/ml) also significantly suppresses neutrophil apoptosis, as evidenced by morphology and annexin V binding (10, 24). No significant change was found in the percentage of neutrophil apoptosis, as evidenced by morphology or annexin V binding, after treatment with EMA or CAM (10 μg/ml) for 24 h, irrespective of the presence of LPS (Fig. 1). None of the other antibiotics, which included JM, AMP, and CCL, were found to alter the percentage of neutrophil apoptosis (data not shown). MG-132, a potent inhibitor of NF-κB activation, significantly suppressed IL-8 production by LPS-stimulated neutrophils (24) compared with that by untreated controls (Fig. 2). DEX at clinically achievable levels similarly inhibited IL-8 production of LPS-stimulated neutrophils in a concentration-dependent manner (22). EMA and CAM, at concentrations of 1.0 and 10 μg/ml, demonstrated a slight but significant inhibition of IL-8 production by LPS-stimulated neutrophils at 48 h (Fig. 2B); only at a concentration of 10 μg/ml did EM and CAM each show a significant decrease in IL-8 production at 24 h postincubation (Fig. 2A). JM, which is a 16-member macrolide, AMP, and CCL had no effect on IL-8 production by LPS-stimulated neutrophils (data not shown). No effects by the 14-member macrolides, DEX, or MG-132 were found on the cell viability of LPS-stimulated neutrophils after a 48-h incubation (data not shown). DEX, which has been reported to interfere with the binding of NF-κB to its cognate cis element in vitro (14), suppressed the level of IL-8 mRNA expression of LPS-stimulated neutrophils by 49% compared with that in the control (Fig. 3). Each of the 14-member macrolides at a concentration of 10 μg/ml similarly inhibited the level of IL-8 mRNA expression of LPS-stimulated neutrophils (by 34% for EMA and 43% for CAM).
FIG. 1.
Effects of 14-member macrolides and DEX on neutrophil apoptosis, as estimated by morphology (A) and annexin V binding (B), in the presence (solid bars) or absence (open bars) of LPS. Separated neutrophils were incubated with a 14-member macrolide (10 μg/ml) or DEX (10−7 M; 43 ng/ml), and LPS (1 μg/ml) was then added to the cell suspension. After a 24-h incubation, neutrophils were recovered and analyzed for apoptosis. The control (CTRL) contained 0.1% DMSO in IMDM. Each value is the mean ± standard deviation from three determinations. ∗∗, P < 0.01 (compared with the control in the absence of LPS).
FIG. 2.
Effects of 14-member macrolides, DEX, or MG-132 (10−6 M) on IL-8 production by human neutrophils in the presence of LPS after 24 (A) or 48 (B) h of incubation. Separated neutrophils were incubated with each reagent at the indicated concentrations, and LPS (1 μg/ml) was then added to the cell suspension. After a 24- or 48-h incubation, IL-8 levels in culture supernatants were determined. The control (CTRL) contained 0.1% DMSO in IMDM. Each value is the mean ± standard deviation from three determinations. ∗∗, P < 0.01; ∗, P < 0.05 (compared with the control in the presence of LPS).
FIG. 3.
Effects of EMA (10 μg/ml), CAM (10 μg/ml), and DEX (10−7 M; 43 ng/ml) on IL-8 gene expression in LPS-stimulated human neutrophils. IL-8 gene expression of uncultured neutrophils without LPS stimulation was also examined as a negative control. The control medium (CTRL) contained 0.1% DMSO in IMDM. Results shown are IL-8 mRNA transcript levels (upper panel) and control G3PDH mRNA transcript levels (lower panel) in human neutrophils. Bars in graph represent densitometric units obtained from an autoradiogram by Northern blot analysis and expressed as the ratios of IL-8 mRNA transcript levels to G3PDH mRNA transcript levels.
A previous in vitro study demonstrated that macrolides, including EM, shortened neutrophil survival by accelerating apoptosis (2). The authors suggested that enhancement of neutrophil apoptosis by EM may provide a procedure for improving neutrophil clearance in the airways of CAD patients, contributing to the clinical effect of low-dose, long-term EM therapy. This study, however, involved only morphological evaluation by electron microscopy of the effects of EM on spontaneous apoptosis of cultured neutrophils in the absence of serum and failed to demonstrate the effects of EM on the apoptosis of activated neutrophils with a longer life span. In the present study, none of the antibiotics tested, including 14-member macrolides, altered the percentage of spontaneous apoptosis in the presence of heat-inactivated serum (Fig. 1A). Differences in the methods used for the evaluation of neutrophil apoptosis may account for the discrepancy between the observed effects of EM on the spontaneous apoptosis of neutrophils. More importantly, 14-member macrolides had no effect on the percentage of LPS-stimulated neutrophils for which phosphatidylserine was exposed on the surfaces (Fig. 1B). Our present data, therefore, suggest that 14-member macrolides do not promote neutrophil apoptosis in the airways.
Previous investigators have demonstrated the down-regulation of IL-8 mRNA expression of stimulated bronchial epithelial cells by 14-member macrolides (1, 21). On the other hand, our goal was to underscore the importance of IL-8 production by activated neutrophils in the airways of patients with CAD (17; Yoshimine et al., Am. J. Respir. Crit. Care Med. 161:A338, 2000). In this study, we demonstrated that EM or CAM at clinically achievable levels led to mild suppression of IL-8 production by LPS-stimulated neutrophils (15), although the inhibitory effects of 14-member macrolides on IL-8 production were much weaker than that of DEX. We also found down-regulation of IL-8 mRNA expression by 14-member macrolides in LPS-stimulated neutrophils, although the concentration of drugs used was slightly higher than the clinically achievable level. In addition, long-term administration of DEX, but not of a 14-member macrolide, may impair pulmonary defense through the suppression of inducible nitric oxide synthase and tumor necrosis factor alpha, which are critical for defense against bacterial infections (18). Collectively, these data support the view that low-dose, long-term therapy with a 14-member macrolide may be effective for CAD by suppressing IL-8 production by activated neutrophils but not by accelerating apoptosis in these cells.
REFERENCES
- 1.Abe, S., H. Nakamura, S. Inoue, H. Takeda, H. Saito, S. Kato, N. Mukaida, K. Matsushima, and H. Tomoike. 2000. Interleukin-8 gene repression by clarithromycin is mediated by the activator protein-1 binding sites in human bronchial epithelial cells. Am. J. Respir. Cell. Mol. Biol. 22:51-60. [DOI] [PubMed] [Google Scholar]
- 2.Aoshiba, K., A. Nagai, and K. Konno. 1995. Erythromycin shortens neutrophil survival by accelerating apoptosis. Antimicrob. Agents Chemother. 39:872-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cox, G., and R. Austin. 1997. Dexamethasone-induced suppression of apoptosis in human neutrophils requires continuous stimulation of new protein synthesis. J. Leukoc. Biol. 61:224-230. [DOI] [PubMed] [Google Scholar]
- 4.Fadok, V. A., D. L. Bratton, D. M. Rose, A. Pearson, R. A. B. Ezekewitz, and P. M. Henson. 2000. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature 405:85-90. [DOI] [PubMed] [Google Scholar]
- 5.Haslett, C. 1999. Granulocyte apoptosis and its role in the resolution and control of lung inflammation. Am. J. Respir. Crit. Care Med. 160:S5-S11. [DOI] [PubMed] [Google Scholar]
- 6.Homma, H., A. Yamanaka, S. Tanimoto, M. Tamura, Y. Chijimatsu, S. Kira, and T. Izumi. 1983. Diffuse panbronchiolitis: a disease of the transitional zone of the lung. Chest 83:63-69. [DOI] [PubMed] [Google Scholar]
- 7.Kadota, J., O. Sakito, S. Kohno, H. Sawa, H. Mukae, H. Oda, K. Kawakami, K. Fukushima, K. Hiratani, and K. Hara. 1993. A mechanism of erythromycin treatment in patients with diffuse panbronchiolitis. Am. Rev. Respir. Dis. 147:153-159. [DOI] [PubMed] [Google Scholar]
- 8.Kudoh, S., T. Uetake, K. Hagiwara, L. H. Hus, H. Limura, and Y. Sugiyama. 1987. Clinical effect of low-dose, long-term erythromycin chemotherapy on diffuse panbronchiolitis. Jpn. J. Thorac. Dis. 25:632-642. [PubMed] [Google Scholar]
- 9.Kudoh, S., A. Azuma, M. Yamamoto, T. Izumi, and M. Ando. 1998. Improvement of survival in patients with diffuse panbronchiolitis treated with low-dose erythromycin. Am. J. Respir. Crit. Care Med. 157:1829-1832. [DOI] [PubMed] [Google Scholar]
- 10.Lee, A., M. K. B. Whyte, and C. Haslett. 1993. Inhibition of apoptosis and prolongation of neutrophil functional longevity by inflammatory mediators. J. Leukoc. Biol. 54:283-288. [PubMed] [Google Scholar]
- 11.Matute-Bello, G., W. C. Liles, F. Radella II, K. P. Steinberg, J. T. Ruzinski, M. Jonas, E. Y. Chi, L. D. Hudson, and T. R. Martin. 1997. Neutrophil apoptosis in the acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 156:1969-1977. [DOI] [PubMed] [Google Scholar]
- 12.Mevorach, D., J. O. Mascarenhas, D. Gershov, and K. B. Elkon. 1998. Complement-dependent clearance of apoptotic cells by human macrophages. J. Exp. Med. 188:2313-2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morimoto, K., H. Amano, F. Sonoda, M. Baba, M. Senba, H. Yoshimine, H. Yamamoto, T. Ii, K. Oishi, and T. Nagatake. 2001. Alveolar macrophages that phagocytose apoptotic neutrophils produce hepatocyte growth factor during bacterial pneumonia in mice. Am. J. Respir. Cell. Mol. Biol. 24:608-615. [DOI] [PubMed] [Google Scholar]
- 14.Mukaida, N., M. Morita, Y. Ishikawa, N. Rice, S. Okamoto, T. Kasahara, and K. Matsushima. 1994. Novel mechanism of glucocorticoid-mediated gene repression. Nuclear factor-κB is target for glucocorticoid-mediated interleukin-8 gene repression. J. Biol. Chem. 269:13289-13295. [PubMed] [Google Scholar]
- 15.Nagai, H., H. Shishido, R. Yoneda, E. Yamaguchi, A. Tamura, and A. Kurashima. 1991. Long-term low-dose administration of erythromycin to patients with diffuse panbronchiolitis. Respiration 58:145-149. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura, H., K. Yoshimura, N. G. McElvaney, and R. G. Crystal. 1992. Neutrophil elastase in respiratory epithelial lining fluid of individuals with cystic fibrosis induces interleukin-8 gene expression in human bronchial epithelial cell lines. J. Clin. Investig. 89:1478-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oishi, K., F. Sonoda, S. Kobayashi, A. Iwagaki, T. Nagatake, K. Matsushima, and K. Matsumoto. 1994. Role of interleukin-8 (IL-8) and an inhibitory effect of erythromycin on IL-8 release in the airways of patients with chronic airway diseases. Infect. Immun. 62:4145-4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Satoh, S., K. Oishi, A. Iwagaki, M. Senba, T. Akaike, M. Akiyama, N. Mukaida, K. Matsushima, and T. Nagatake. 2001. Dexamethasone impairs pulmonary defence against Pseudomonas aeruginosa through suppressing iNOS gene expression and peroxynitrite production in mice. Clin. Exp. Immunol. 126:266-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savill, J., N. Hogg, Y. Ren, and C. Haslett. 1992. Thrombospondin cooperates with CD36 and the vitronectin receptor in macrophage recognition of neutrophils undergoing apoptosis. J. Clin. Investig. 90:1513-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takeda, H., H. Miura, M. Kawahira, H. Kobayashi, S. Otomo, and S. Nakaike. 1989. Long-term administration study on TE-031 (A-56268) in the treatment of diffuse panbronchiolitis. J. Jpn. Assoc. Infect. Dis. 63:71-78. (In Japanese.) [DOI] [PubMed] [Google Scholar]
- 21.Takizawa, H., M. Desaki, T. Ohtoshi, S. Kawasaki, T. Kohyama, M. Sato, M. Tanaka, T. Kasama, K. Kobayashi, J. Nakajima, and K. Ito. 1997. Erythromycin modulates IL-8 expression in normal and inflamed human bronchial epithelial cells. Am. J. Respir. Crit. Care Med. 156:266-271. [DOI] [PubMed] [Google Scholar]
- 22.Tsuei, S. E, R. G. Moore, J. J. Ashley, and W. G. McBride. 1979. Disposition of synthetic glucocorticoids. I. Pharmacokinetics of dexamethasone in healthy adults. J. Pharmacokin. Biopharm. 7:249-264. [DOI] [PubMed] [Google Scholar]
- 23.Wang, A., A. Wada, K. Yahiro, T. Nomura, Y. Fujii, K. Okamoto, Y. Mizuta, S. Kohno, J. Moss, and T. Hirayama. 1999. Identification and characterization of the Aeromonas sobria hemolysin glycoprotein receptor on intestine 407 cells. Microb. Pathog. 27:215-221. [DOI] [PubMed] [Google Scholar]
- 24.Ward, C., E. R. Chilvers, M. F. Lawson, J. G. Pryde, S. Fujihara, S. N. Farrow, C. Haslett, and A. G. Rossi. 1999. NF-κB activation is a critical regulator of human granulocyte apoptosis in vitro. J. Biol. Chem. 274:4309-4318. [DOI] [PubMed] [Google Scholar]
- 25.Watson, R. W. G., O. D. Rotstein, A. B. Nathens, J. Parodo, and J. C. Marshall. 1997. Neutrophil apoptosis is mediated by endothelial transmigration and adhesion molecule engagement. J. Immunol. 158:945-953. [PubMed] [Google Scholar]
- 26.Yang, D., S. Suzuki, L. J. Hao, Y. Fujii, A. Yamauchi, M. Yamamoto, M. Nakamura, and A. Kumatori. 2000. Eosinophil-specific regulation of gp91(phox) gene expression by transcription factors GATA-1 and GATA-2. J. Biol. Chem. 275:9425-9432. [DOI] [PubMed] [Google Scholar]