Abstract
We compared the efficacies of quinupristin-dalfopristin (Q-D; 30 mg/kg of body weight every 8 h) and vancomycin (60 mg/kg twice daily), alone or in combination with rifampin (10 mg/kg twice daily), in a rabbit model of methicillin-resistant Staphylococcus aureus knee prosthesis infection. In contrast to vancomycin, Q-D significantly reduced the mean log10 CFU per gram of bone versus that for the controls. The combination of rifampin with either Q-D or vancomycin was significantly more effective than monotherapy.
Infection with methicillin-resistant staphylococci is becoming more frequent among those with implanted orthopedic devices (16), and these organisms are often resistant to many commonly used antibiotics. Glycopeptide antibiotics are usually used as first-line therapy for prosthesis infections. However, effective alternative regimens are urgently needed because the efficacies of these compounds may not be optimal (20, 28) and glycopeptide-intermediate Staphylococcus aureus and S. epidermidis strains are emerging, probably as a consequence of the overuse or the inappropriate use of glycopeptide antibiotics (23, 26).
Quinupristin-dalfopristin (Q-D) has been shown to be as effective as vancomycin against erythromycin-susceptible methicillin-resistant S. aureus (MRSA) strains in an experimental model of endocarditis (9, 10) and was recently shown to be highly active against staphylococci growing in biofilms in vitro, while glycopeptides showed only poor activity (13).
In the present study, we compared the efficacies of Q-D and vancomycin, alone and in combination with rifampin, using a rabbit model of experimental MRSA knee prosthesis infection that closely mimics MRSA infections in humans (2).
(This work was presented in part at the 40th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Ontario, Canada, 17-20 Sept. 2000 [A. Saleh-Mghir, N. Ameur, L. Massias, C. Feger, C. Carbon, and A. C. Crémieux, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 994, 2000].)
An erythromycin-susceptible MRSA strain, HM 1054, was used in this study. The MICs were determined by the broth macrodilution method (19). Time-kill experiments were performed in brain heart infusion (BHI) broth containing Q-D (2.5 μg/ml) or vancomycin (5 μg/ml), either alone or in combination with rifampin (0.325 μg/ml), and an initial inoculum of 106 CFU of HM 1054 per ml. After 0, 3, 6, and 24 h of incubation, 0.1-ml samples were subcultured on BHI agar for determination of the numbers of CFU.
The model of knee prosthesis infection has previously been described in detail (2). Briefly, a partial knee replacement with a tibial component (Silastic great toe implant HP; Swanson Design; Dow-Corning France, S.A.) was performed on the right knees of the rabbits by an orthopedic surgeon. Immediately after surgery, the animals were inoculated with 5 × 107 CFU of MRSA in 0.5 ml, injected into the knee close to the prosthesis. Four days after infection (day 4), the following intramuscular (i.m.) injection regimens were started: Q-D (30 mg/kg of body weight every 8 h) or vancomycin (60 mg/kg twice daily [b.i.d.]), either alone or in combination with rifampin (10 mg/kg b.i.d.). Each regimen was administered for 7 days. The animals were killed 3 days after the end of therapy (day 14) to allow bacterial regrowth following the end of therapy but to avoid the persistence of residual antibiotic in the bone. Untreated control rabbits were also killed on day 14. For quantitative bacterial counts, the upper third of the tibia (length, 3 cm), including compact bone and marrow, was collected, frozen in liquid nitrogen, and crushed in an automatic pulverizer (Spex 6700; Freezer/Mill Industries Inc., Metechen, N.J.). The pulverized bone was suspended in 10 ml of sterile saline, and serial dilutions were made and plated on Trypticase soy agar. After overnight incubation at 37°C, the number of viable organisms was determined. The results are expressed as the mean ± standard deviation (SD) log10 CFU per gram of bone.
Portions (0.1 ml) of each undiluted bone homogenate were also plated on BHI agar containing each antibiotic at two and four time the MIC in order to detect mutant bacteria showing antibiotic resistance after 48 h of incubation.
Plasma antibiotic levels were determined in uninfected rabbits. Plasma Q-D concentrations were assayed microbiologically. The limits of quantification were 0.10 and 0.25 μg/ml for quinupristin and dalfopristin, respectively, and the coefficients of variation of the assay were 4.4 and 2.5% for quinupristin and dalfopristin, respectively. Vancomycin concentrations were determined by fluorescence polarization immunoassay (TDX; Abbott, Rungis, France), and the lower limit of detection was 0.6 μg/ml. Plasma rifampin concentrations were determined by high-performance liquid chromatography coupled with UV detection, and the lower limit of detection was 0.1 μg/ml.
Bacterial densities in bone were compared between the experimental groups by analysis of variance, followed by Scheffe's test for multiple comparisons. The results are expressed as means ± SDs. A P value of <0.05 was considered significant.
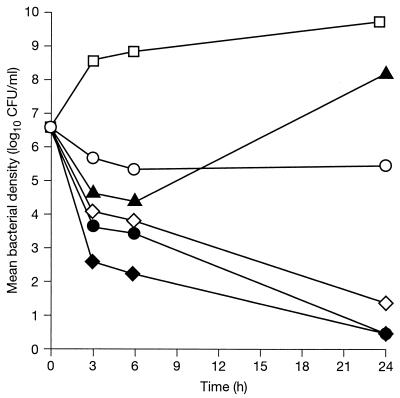
The MICs of Q-D, vancomycin, and rifampin were 0.5, 1, and 0.06 μg/ml, respectively. The in vitro killing curves for the drugs at five times their MICs (Fig. 1) showed that Q-D had greater bactericidal activity than vancomycin. Rifampin was highly active during the first 6 h but then lost activity, presumably due to the emergence of resistant mutants. However, the use of rifampin with Q-D or vancomycin increased the killing rates of both Q-D and vancomycin by approximately 2 log10 CFU at 6 h in each case, and this increase was sustained for the full 24 h of incubation.
FIG. 1.
In vitro killing curves for methicillin-resistant S. aureus strain HM 1054, using different antibacterial agents and combinations, all at five times the MIC. □, control; ◊, Q-D; ○, vancomycin; ▴, rifampin; ⧫, Q-D plus rifampin; •, vancomycin plus rifampin.
All control animals were infected with HM 1054 and exhibited positive prosthesis smear cultures and a mean bacterial count of 6.3 ± 0.6 log10 CFU/g of bone (Table 1). Only 1 of 12 animals in the Q-D-treated group had sterile bone; however, the mean bone bacterial density was significantly reduced compared with that for the control animals (P < 0.01). None of the animals in the vancomycin-treated group had sterile bone, and the bacterial count in bone was not significantly different from that in control animals (P = 0.175). The mean bacterial counts were not significantly different between the Q-D- and vancomycin-treated animals.
TABLE 1.
Effect of antibiotic treatment on experimental methicillin-resistant S. aureus prosthetic knee infection in rabbits
| Treatmenta | No. of rabbits | No. of rabbits with sterile bone | Mean ± SD bacterial count (log10 CFU/g of bone) |
|---|---|---|---|
| None | 9 | 0 | 6.3 ± 0.6 |
| Q-D | 12 | 1 | 4.7 ± 1.3b |
| Vancomycin | 10 | 0 | 5.1 ± 0.8 |
| Q-D + rifampin | 10 | 10 | 1.8 ± 0.1b, c |
| Vancomycin + rifampin | 11 | 5 | 2.9 ± 1.5b, c |
Rabbits were treated i.m. for 7 days with either Q-D (30 mg/kg every 8 h) or vancomycin (60 mg/kg b.i.d.) alone or in combination with rifampin (10 mg/kg b.i.d.).
Significantly different from untreated controls (P < 0.01).
Significantly different from vancomycin and Q-D monotherapy groups (P < 0.01).
The combination of Q-D plus rifampin or the combination of vancomycin plus rifampin was significantly more effective than monotherapy. In the group treated with vancomycin plus rifampin, 5 of 11 animals had sterile bone, and in the group treated with Q-D plus rifampin, 10 of 10 animals had sterile bone. For both groups, the mean bacterial counts were significantly lower than those for the untreated controls and the groups treated with either agent alone (Table 1). Mutant bacteria resistant to rifampin were detected in 5 of 11 animals treated with vancomycin plus rifampin but in 0 of 10 animals treated with Q-D plus rifampin. No Q-D-resistant strain emerged in any of the animals treated with this agent.
Plasma antibiotic levels are shown in Table 2.
TABLE 2.
Plasma antibiotic concentrations in rabbitsa
| Antibiotic | Peak concn (μg/ml) | Trough concn (μg/ml) |
|---|---|---|
| Q-D | 1.10 ± 0.30 (1 h)/ 1.86 ± 0.50 (1 h) | 0.18 ± 0.50 (12 h)/ 0.25 (12 h) |
| Vancomycin | 34.4 ± 14.3 (1 h) | 18.5 ± 4.4 (12 h) |
| Rifampin | 9.70 ± 0.7 (2 h) | 0.23 ± 0.05 (8 h) |
The times in parentheses are the times to the peak or trough concentration.
Bacterial infection associated with prosthetic joints is a relatively rare but severe complication that occurs in up to 2.5% of knee arthroplasty procedures (3, 12, 29). The experimental model used in the present study reproduces a prosthetic knee infection similar to that observed in humans and is suitable for the comparative evaluation of antibiotic therapies (2, 6, 25). S. aureus and S. epidermidis are the major pathogens that cause postoperative prosthetic joint infections (3, 15, 21), and an increasing proportion of isolates of these pathogens are resistant to methicillin and other antibiotics (7, 11, 17, 24), including those commonly used for the treatment of bone infections, such as the fluoroquinolones (14). The glycopeptides, including teicoplanin and vancomycin, have been the only agents that have consistently been shown to have in vitro activity against these multidrug-resistant staphylococci. However, these agents were poorly effective in a rabbit model of S. aureus osteomyelitis (22), despite their good penetration into bone and their low MICs, as determined under conventional aerobic conditions. Similarly, using a tissue-cage model of S. aureus foreign-body infection, Zimmerli et al. (31) obtained only very low cure rates with glycopeptide monotherapy. In the present study, vancomycin monotherapy failed to sterilize bone in any animal and did not significantly reduce the mean bacterial count in bone. Q-D monotherapy sterilized only 1 of 12 animals but did produce a significant reduction in the mean bacterial count. By contrast, the combination of rifampin plus Q-D led to bone and prosthesis sterilization in all (10 of 10) animals tested. One limitation of this result could be the persistence of residual viable bacteria in a bioflim adherent to the device itself. Other investigators (5, 32) have shown that despite an apparent radical cure by use of rifampin in combination with another agent, viable organisms could be detached when the foreign body was sonicated and attached bacteria were recovered. However, rifampin in combination with other antibacterial agents has been demonstrated to have good efficacy in experimental models of foreign-body infections (31) and in orthopedic implant-related infections in patients (27). The efficacy of rifampin as monotherapy for an active infection is limited by the emergence of resistant mutants, especially during long-term therapy (18). The emergence of rifampin resistance during combination therapy with rifampin and vancomycin has been reported clinically (4, 8) and was observed in 5 of 11 animals treated with rifampin plus vancomycin in the present study. By contrast, resistance to rifampin did not emerge in any of the 10 animals treated with rifampin plus Q-D.
The MRSA strain used in the present study (strain HM 1054) was susceptible to rifampin and erythromycin. As shown in experimental endocarditis (30), the in vivo synergistic and bactericidal activities of rifampin plus Q-D are related to the absence of resistance to quinupristin. In a recent study of 4,644 S. aureus strains isolated from a single hospital in France between 1993 and 1996, approximately 25% were resistant to rifampin (MICs, ≥8 μg/ml) (1), and more than 50% of MRSA isolates are resistant to quinupristin.
The present results suggest that combination therapy with Q-D and rifampin is more effective than conventional therapy for experimental quinupristin- and rifampin-susceptible MRSA prosthetic joint infection.
Acknowledgments
MRSA strain HM 1054 was isolated from the blood of a patient with sepsis at the Henri Mondor Hospital, Créteil, France, and was kindly provided by Roland Leclercq. We also thank Ortho Technique, Créteil, France, for providing the silastic toe implants.
This work was supported by a grant from Aventis Pharma, Paris, France.
REFERENCES
- 1.Aubry-Damon, H., C.-J. Soussy, and P. Courvalin. 1998. Characterization of mutations in the rpoB gene that confer rifampin resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 42:2590-2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belmatoug, N., A. C. Crémieux, R. Bleton, A. Volk, A. Saleh-Mghir, M. Grossin, L. Garry, and C. Carbon. 1996. A new model of experimental prosthetic joint infection due to methicillin-resistant Staphylococcus aureus: a microbiologic, histopathologic, and magnetic resonance imaging characterization. J. Infect. Dis. 174:414-417. [DOI] [PubMed] [Google Scholar]
- 3.Bengtson, S. 1993. Prosthetic osteomyelitis with special reference to the knee: risks, treatment and costs. Ann. Med. 25:523-529. [PubMed] [Google Scholar]
- 4.Burnie, J., R. Matthews, A. Jiman-Fatami, P. Gottardello, S. Hodgetts, and S. D'Arcy. 2000. Analysis of 42 cases of septicemia caused by an epidemic strain of methicillin-resistant Staphylococcus aureus: evidence of resistance to vancomycin. Clin. Infect. Dis. 31:684-689. [DOI] [PubMed] [Google Scholar]
- 5.Chuard, C., M. Herrmann, P. Vaudaux, F. A. Waldvogel, and D. P. Lew. 1991. Successful therapy of experiment chronic foreign-body infection due to methicillin-resistant Staphylococcus aureus by antimicrobial combinations. Antimicrob. Agents Chemother. 35:2611-2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crémieux, A. C., A. Saleh-Mghir, R. Bleton, M. Manteau, N. Belmatoug, L. Massias, L. Garry, N. Sales, B. Mazière, and C. Carbon. 1996. Efficacy of sparfloxacin and autoradiographic pattern of diffusion of [14C]sparfloxacin in an experimental Staphylococcus aureus joint prosthesis infection. Antimicrob. Agents Chemother. 40:2111-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dowzicky, M., H. L. Nadler, C. Feger, G. Talbot, F. Bompart, and M. Pease. 1998. Evaluation of in vitro activity of quinupristin/dalfopristin and comparator antimicrobial agents against worldwide clinical trial and other laboratory isolates. Am. J. Med. 104(Suppl. 5A):34S-42S. [DOI] [PubMed] [Google Scholar]
- 8.Eng, R. H., S. M. Smith, M. Tillem, and C. Cherubin. 1985. Rifampin resistance. Development during the therapy of methicillin-resistant Staphylococcus aureus infection. Arch. Intern. Med. 145:146-148. [DOI] [PubMed] [Google Scholar]
- 9.Entenza, J. M., H. Drugeon, M. P. Glauser, and P. Moreillon. 1995. Treatment of experimental endocarditis due to erythromycin-susceptible or -resistant methicillin-resistant Staphylococcus aureus with RP 59500. Antimicrob. Agents Chemother. 39:1419-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fantin, B., R. Leclercq, Y. Merle, L. Saint-Julien, C. Veyrat, J. Duval, and C. Carbon. 1995. Critical influence of resistance to streptogramin B-type antibiotics on activity of RP 59500 (quinupristin-dalfopristin) in experimental endocarditis due to Staphylococcus aureus. Antimicrob. Agents Chemother. 39:400-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fluit, A. C., M. E. Jones, F.-J. Schmitz, J. Acar, R. Gupta, J. Verhoef, and the SENTRY Participants Group. 2000. Antimicrobial susceptibility and frequency of occurrence of clinical blood isolates in Europe from the SENTRY Antimicrobial Surveillance Program, 1997 and 1998. Clin. Infect. Dis. 30:454-460. [DOI] [PubMed] [Google Scholar]
- 12.Gaine, W. J., N. A. Ramamohan, N. A. Hussein, M. G. Hullin, and S. W. McCreath. 2000. Wound infection in hip and knee arthroplasty. J. Bone Joint Surg. Br. 82:561-565. [DOI] [PubMed] [Google Scholar]
- 13.Gander, S., and R. Finch. 2000. The effects of exposure at constant (1 h) or exponentially decreasing concentrations of quinupristin/dalfopristin on biofilms of gram-positive bacteria. J. Antimicrob. Chemother. 46:61-67. [DOI] [PubMed] [Google Scholar]
- 14.Gentry, L. O. 1991. Oral antimicrobial therapy for osteomyelitis. Ann. Intern. Med. 114:986-987. [DOI] [PubMed] [Google Scholar]
- 15.Ivey, F. M., C. A. Hicks, J. H. Calhoun, and J. T. Mader. 1990. Treatment options for infected knee arthroplasties. Rev. Infect. Dis. 12:468-478. [DOI] [PubMed] [Google Scholar]
- 16.James, P. J., I. A. Butcher, E. R. Gardner, and D. L. Hamblen. 1994. Methicillin-resistant Staphylococcus epidermidis in infection of hip arthroplasties. J. Bone Joint Surg. Br. 76:725-727. [PubMed] [Google Scholar]
- 17.Jones, R. N., C. H. Ballow, D. J. Biedenbach, J. A. Deinhart, and J. J. Schentag. 1998. Antimicrobial activity of quinupristin-dalfopristin (RP 59500, Synercid®) tested against over 28,000 recent clinical isolates from 200 medical centers in the United States and Canada. Diagn. Microbiol. Infect. Dis. 30:437-451. [DOI] [PubMed] [Google Scholar]
- 18.Mandell, G. L., and D. R. Moorman. 1980. Treatment of experimental staphylococcal infections: effect of rifampin alone and in combination on development of rifampin resistance. Antimicrob. Agents Chemother. 17:658-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Committee for Clinical Laboratory Standards. 1985. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard. Publication M7-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 20.Norden, C. W. 1988. Lessons learned from animal models of osteomyelitis. J. Infect. Dis. 40:103-110. [DOI] [PubMed] [Google Scholar]
- 21.Norden, C. W., J. D. Nelson, J. T. Mader, and G. B. Calandra. 1992. Evaluation of new anti-infection drugs for the treatment of infection of prosthetic hip joint. Clin. Infect. Dis. 15:S177-S181. [DOI] [PubMed] [Google Scholar]
- 22.Norden, C. W., K. Nidereiter, and E. M. Shinners. 1986. Treatment of experimental chronic osteomyelitis due to Staphylococcus aureus with teicoplanin. Infection 14:136-138. [DOI] [PubMed] [Google Scholar]
- 23.Perl, T. M. 1999. The threat of vancomycin resistance. Am. J. Med. 106(5A):26S-37S. [DOI] [PubMed] [Google Scholar]
- 24.Pfaller, M. A., R. N. Jones, G. V. Doern, K. Kugler, and the SENTRY Participants Group. 1998. Bacterial pathogens isolated from patients with bloodstream infection: frequencies of occurrence and antimicrobial susceptibility patterns from the SENTRY Antimicrobial Surveillance Program (United States and Canada, 1997). Antimicrob. Agents Chemother. 42:1762-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saleh-Mghir, A., A. C. Crémieux, R. Bleton, F. Ismael, M. Manteau, S. Dautrey, L. Massias, L. Garry, N. Sales, B. Maziere, and C. Carbon. 1998. Efficacy of teicoplanin and autoradiographic diffusion pattern of [14C]teicoplanin in experimental Staphylococcus aureus infection of joint prostheses. Antimicrob. Agents Chemother. 42:2830-2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith, T. L., M. L. Pearson, K. R. Wilcox, C. Cruz, M. V. Lancaster, B. Robinson-Dunn, F. C. Tenover, M. J. Zervos, J. D. Band, E. White, and W. R. Jarvis, for the Glycopeptide-Intermediate Staphylococcus aureus Working Group. 1999. Emergence of vancomycin resistance in Staphylococcus aureus. N. Engl. J. Med. 340:493-501. [DOI] [PubMed] [Google Scholar]
- 27.Widmer, A. F., A. Gaechter, P. E. Ochsner, and W. Zimmerli. 1992. Antimicrobial treatment of orthopedic implant-related infections with rifampin combinations. Clin. Infect. Dis. 14:1251-1253. [DOI] [PubMed] [Google Scholar]
- 28.Wood, M. J. 1999. Chemotherapy for gram-positive nosocomial sepsis. J. Chemother. 11:446-452. [DOI] [PubMed] [Google Scholar]
- 29.Wymenga, A. B., J. R. van Horn, A. Theeuwes, H. L. Muytjens, and T. J. Slooff. 1992. Perioperative factors associated with septic arthritis after arthroplasty. Prospective multicenter study of 362 knee and 2,651 hip operations. Acta Orthotop. Scand. 63:665-671. [DOI] [PubMed] [Google Scholar]
- 30.Zarrouk, V., B. Bozdogan, R. Leclercq, L. Garry, C. Feger, C. Carbon, and B. Fantin. 2001. Activities of the combination of quinupristin-dalfopristin with rifampin in vitro and in experimental endocarditis due to Staphylococcus aureus strains with various phenotypes of resistance to macrolides-lincosamide-streptogramin antibiotics. Antimicrob. Agents Chemother. 45:1244-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zimmerli, W., A. F. Widmer, M. Blatter, R. Frei, P. E. Ochsner, et al. 1998. Role of rifampin for treatment of orthopedic implant-related Staphylococcus aureus: a randomized controlled trial. JAMA 279:1537-1541. [DOI] [PubMed] [Google Scholar]
- 32.Zimmerli, W., R. Frei, A. F. Widmer, and Z. Rajacic. 1994. Microbiological tests to predict treatment outcome in experimental device-related infections due to Staphylococcus aureus. J. Antimicrob. Chemother. 33:959-967. [DOI] [PubMed] [Google Scholar]