Abstract
Several cyclopentane inhibitors of influenza virus neuraminidase that have inhibitory activities in tissue culture similar to those of zanamivir and oseltamivir have recently been described. These new inhibitors have been examined for efficacy against a virulent H3N2 influenza virus when administered orally to infected ferrets. Preliminary studies indicated that oral administration of BCX-1923, BCX-1827, or BCX-1812 (RWJ-270201) at a dose of 5 or 25 mg/kg of body weight was active in ferrets in reducing respiratory and constitutional signs and symptoms, but these antivirals affected virus titers in the upper and lower respiratory tracts only marginally. Of the three compounds, BCX-1812 seemed to be the most efficacious and was examined further at higher doses of 30 and 100 mg/kg. These doses significantly reduced peak virus titers in nasal washes and total virus shedding as measured by areas under the curve. Virus titers in lung homogenates were also reduced compared to those in controls, but the difference was not statistically significant. As was observed with BCX-1812 at lower doses, the nasal inflammatory cellular response, fever, and nasal signs were reduced while ferret activity was not, with activity remaining similar to uninfected animals.
Influenza, a pandemic viral respiratory disease, results in high morbidity and mortality in both humans and domestic animals. Human influenza is predominantly an upper respiratory tract infection, although influenza pneumonia is a rare complication. Protection can be afforded by annual immunization with a vaccine consisting of inactivated virus or virion subunits, but limitations on vaccine efficacy, design, uptake, and production have stimulated the search for antiviral drugs.
Over the years, a number of compounds with anti-influenza virus activity have been produced, including nucleoside analogues such as ribavirin (23), 2′-deoxy-2′-fluoroguanosine (33), and 1,3,4-thiadiazol-2-ylcyanamide (LY217896) (10), but these have not reached the clinic because of toxicity. Amantadine and its analogue rimantadine, inhibitors of virus M2 ion channel activity and hence of uncoating (12), have reached the clinic, but they are less efficacious (they are active only against influenza A virus), have adverse side effects, and rapidly generate drug-resistant strains (6, 11, 13) which are as virulent as amantadine-sensitive viruses (28).
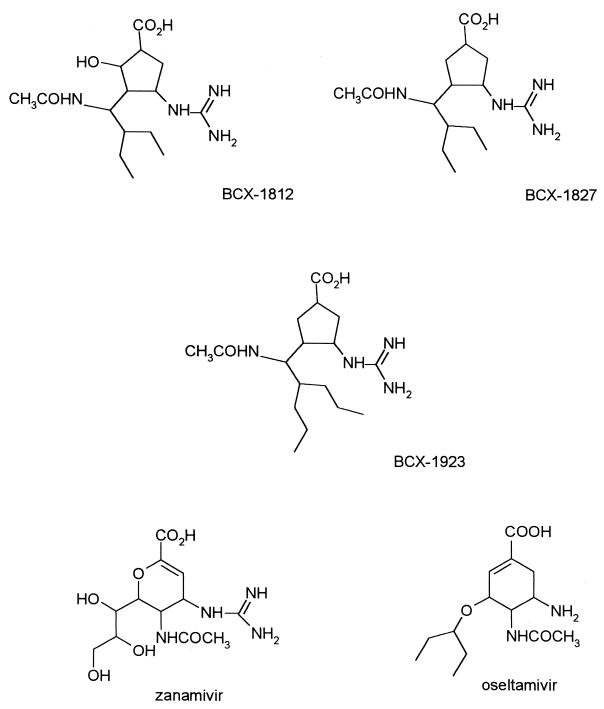
Novel drugs active against the influenza virus neuraminidase from both type A and B strains have recently been described. Some of these have now been approved for use in patients, thereby offering an alternative antiviral strategy (structures are shown in Fig. 1). On the basis of X-ray crystallographic studies of the influenza virus neuraminidase cocrystallized with sialic acid and the unsaturated sialic acid analogue Neu5Ac2en (8, 34, 35), several sialic acid analogues have been synthesized and tested as potential inhibitors of the enzyme. Zanamivir (GG167; 4-guanidino-Neu5Ac2en) is a selective inhibitor of influenza A and B virus neuraminidases. The efficacy of zanamivir has been demonstrated with animal models of influenza virus infection (21, 35) and in studies with humans (14), but its poor bioavailability and rapid elimination from the body necessitate its delivery as a nasal spray (14, 21). Oseltamivir carboxylate, GS4071, a carbocyclic transition state analogue inhibitor of neuraminidase, has potent in vitro inhibitory activity comparable to that of zanamivir (15). Furthermore, oseltamivir (GS4104), an ethyl ester prodrug which is converted to GS4071 in vivo, can be given orally and has been shown to be active in the mouse and ferret models of influenza (16, 18). Based on a rationally designed set of inhibitors, a novel series of cyclopentane derivatives, which are structurally different from sialic acid, has been found to exhibit strong and selective inhibitory effects on influenza virus neuraminidase (2, 3). These compounds, designated BCX-1812 (RWJ-270201), BCX-1827 (RWJ-270204), and BCX-1923, have been shown to inhibit neuraminidase activity from various strains of influenza A virus, with 50% inhibitory concentrations (IC50s) between 0.09 and 1.4 nM and IC50s ranging from 0.82 to 10.8 nM for neuraminidase activity from influenza B virus strains (4). These compounds had 50% effective (virus-inhibitory) concentrations (EC50) ranging between 0.01 and 1.5 μM for 4 H1N1, 12 H3N2, and 2 H5N1 influenza A virus strains and between 0.02 and 3 μM for 5 influenza B virus strains (24, 25) in tissue culture assays. No cytotoxicity was observed with concentrations as high as 1 mM (328 μg/ml), and RWJ-270201 has been shown to be a potent inhibitor of influenza A and B virus infection in mice (24, 25). Here we report the efficacies of these compounds in comparison to those of oseltamivir in the ferret influenza virus model (27).
FIG. 1.
Chemical structures of various neuraminidase inhibitors.
MATERIALS AND METHODS
Materials.
Oseltamivir, BCX-1827, and BCX-1923 were synthesized at BioCryst Pharmaceuticals Inc. (Birmingham, Ala.). BCX-1812 (RWJ-270201) was provided by the R. W. Johnson Pharmaceutical Research Institute (Raritan, N.J.).
Viruses and their assay.
The H3N2 clone 7a of the A/Puerto Rico/8/34-A/England/939/69 reassortant virus system has been described previously (17). Seed stocks were prepared as previously described (29) and titrated in eggs or allantois-on-shell cultures (egg bits) as described previously (29); titers are expressed as 50% egg infectious dose (EID50) or 50% egg bit infectious dose (EBID50). The limit of detection of these assays is 1.5 log10 EID50 or EBID50/ml. Some nasal virus samples were titrated by plaque assay in MDCK cells (1), and these titers are expressed in PFU per milliliter; the limit of detection of this assay is 4 PFU/ml.
Intranasal inoculation of ferrets, collection of nasal washings, and counts of inflammatory cells.
Adult male ferrets, obtained from Highgate Farm, Normanby-by-Spittle, Market Rasen, Lincolnshire, United Kingdom, and in the weight range of 1 to 1.5 kg, received intranasal inoculations with 106 EID50 of clone 7a under ether anesthesia, and nasal washings were performed and inflammatory cell counts were obtained as described previously (30, 31). All studies were carried out in accordance with the United Kingdom, Animals (Scientific Procedures) Act 1986, project license number 40/1715.
Ferret temperatures.
Microchip temperature transponders (Biomedic Data Inc., Seaford, Del.) were implanted subcutaneously into ferrets between 5 and 7 days prior to the experiment, allowing temperatures to be recorded twice daily to determine background temperatures for individual ferrets as described previously (30, 31). Temperatures were recorded at 6- to 12-h intervals during the experiment, and rises in temperature above the preinfection mean for each ferret were determined.
Assessment of signs and activity in the ferrets.
Signs and activity were monitored and assessed in the ferrets as previously described (20).
Lung collection and maceration.
Lung collection and maceration procedures were described by Campbell et al. (9).
Hemagglutination inhibition tests.
Hemagglutination inhibition tests were performed on the sera of ferrets prior to infection to establish the lack of prior immunity of the animals as described by Sweet et al. (29).
Experimental design.
Three to four ferrets, shown to have no serum antibody to clone 7a, were examined for each treatment group, and an untreated but infected group was included in each experiment. Animals were administered their doses orally by placing a syringe over the tongue towards the back of the mouth, allowing the ferrets to easily swallow the compound without stress. Compounds were prepared in the required concentration (e.g., 10 mg/kg body weight) in 1 ml of solution (i.e., 10 mg/ml), and thus volumes for oral doses varied according to the weight of the animal between 1 and 1.5 ml. Doses were given 2 h prior to virus inoculation and then at 12, 24, 36, 48, 60, and 72 h postinfection (hpi). Following virus inoculation, nasal washings were taken and temperatures were recorded every 6 to 12 h at 12, 24, 30, 36, 48, 54, 60, 72, 78, 84, and 96 hpi. On day 4 the animals were sacrificed and their lungs were removed for virus titration.
Pharmacokinetics.
Three nonfasted male ferrets each received a single oral 10-mg/kg gavage dose (1 ml/kg) of BCX-1812 (RWJ-270201) in phosphate-buffered saline. Blood samples (∼0.5 ml) were collected into heparinized tubes via the jugular vein at predose and 0.5, 1, 2, 4, 8, and 24 h postdose. The remaining three nonfasted male ferrets each received a single intravenous (i.v.) 10-mg/kg bolus dose (1 ml/kg) of BCX-1812 into the jugular vein, and blood samples were collected via the opposing jugular vein at predose and 0.1, 0.5, 1, 2, 4, 8, and 24 h postdose. Plasma was separated by centrifugation and stored frozen. Samples were analyzed for plasma BCX-1812 concentrations using a liquid chromatography-mass spectrometry/mass spectrometry assay with a lower limit of quantification of 1 ng/ml. Noncompartmental pharmacokinetic analysis of these data was performed using WinNonlin (version 1.5; Pharsight, Palo Alto, Calif.).
Statistical analysis.
Data were collated and analyzed for statistical significance by the Student's t test.
RESULTS
Effect of oral administration (5 mg/kg) of oseltamivir and BCX-1827 on influenza infection in ferrets.
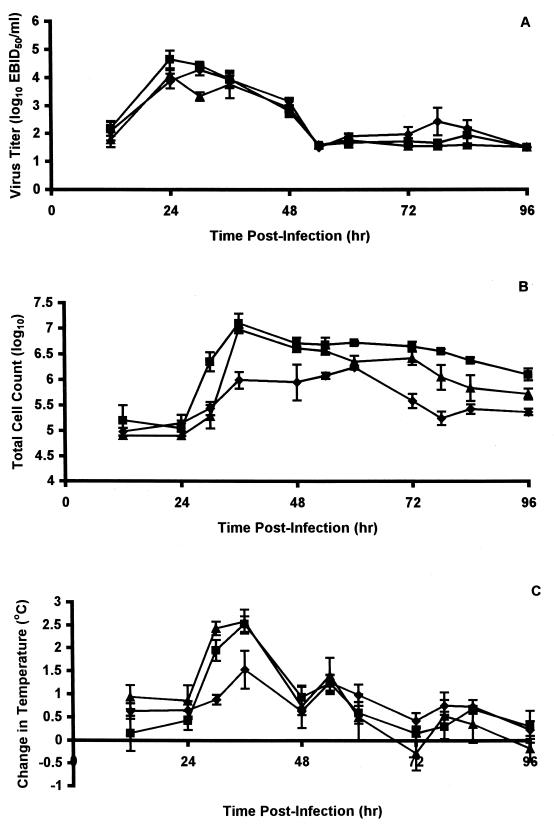
Influenza in ferrets is largely an upper respiratory tract infection with associated respiratory (as monitored by the nasal cellular inflammatory response) and constitutional (as monitored by fever) symptoms, although there is some replication in the lower respiratory tract (26). Thus, in untreated control animals, virus in nasal washes reached mean peak titers of 104.4 to 104.7 EBID50/ml between 24 and 36 hpi and then declined rapidly; a second minor peak of replication was observed between 60 and 96 hpi (Fig. 2A). Animals that were orally administered oseltamivir at 5 mg/kg twice daily also shed virus as early as 12 hpi, with a peak (104.3 EBID50/ml) slightly later (30 hpi), but this peak was less than threefold lower than that observed in untreated animals (Fig. 2A). Virus titers declined rapidly, reaching low levels (101.5 EBID50/ml) by 54 hpi, but again a minor second peak of replication was observed. Animals receiving similar doses of BCX-1827 showed greater inhibition of virus replication, being depressed ∼4-fold at 24 hpi and ∼13-fold at 30 hpi (P < 0.004), although at later times virus titers were similar to those observed in untreated controls (Fig. 2A).
FIG. 2.
Effect of oral administration of compounds on nasal virus titers, nasal inflammatory cell counts, and fever of ferrets infected with influenza virus A (H3N2) virus. Nasal virus titers (A), total numbers of inflammatory cells in nasal washes (B), and changes in temperature relative to baseline temperatures (C) were monitored in ferrets at the indicated times after infection. Ferrets were left untreated (▪) or were treated twice daily with oral 5-mg/kg doses of GS4104 (⧫) or BCX-1827 (▴) for 3 days beginning 2 h prior to infection with influenza virus clone 7a. Values are means ± standard errors of the means (error bars) for three to four animals.
While virus titers seemed to be little affected by treatment with oseltamivir, nasal inflammatory cell counts were considerably suppressed; peak cell counts were delayed (∼24 h) and were significantly (P < 0.0008) lower (approximately sevenfold), and cell counts returned more quickly to near normal levels (Fig. 2B). In contrast, oral treatment of ferrets with BCX-1827 had little effect on the inflammatory response, although the onset of this response was delayed and declined more rapidly at later times (Fig. 2B).
BCX-1827 had similarly little effect on the febrile response, while oseltamivir delayed the febrile response and its peak (Fig. 2C).
While nasal signs (sneezing, nasal discharge, and mouth breathing) induced by clone 7a in untreated animals were relatively mild, both oseltamivir- and BCX-1827-treated animals exhibited little or no nasal signs, and their activities were essentially normal (data not shown).
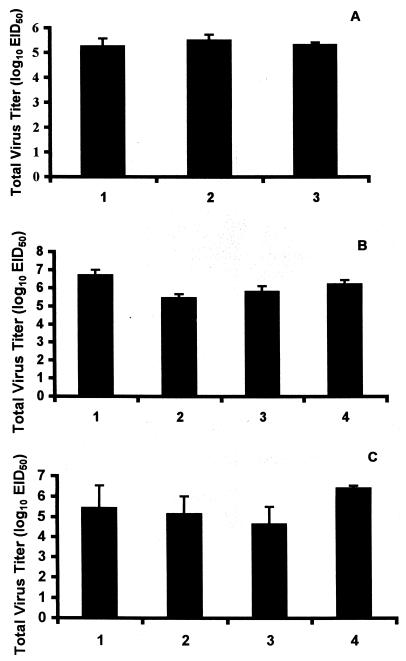
Virus titers in lung 4 days postinfection were not significantly (P > 0.05) reduced in oseltamivir- or BCX-1827-treated ferrets (Fig. 3A).
FIG. 3.
Effect of oral administration of compounds on total virus titers in lung. (A) Ferrets were treated orally with 5-mg/kg doses of GS4014 (bar 1) or BCX-1827 (bar 2) or were untreated (bar 3). (B) Ferrets were treated orally with 25-mg/kg doses of BCX-1827 (bar 1), BCX-1823 (bar 2), or BCX-1812 (bar 3) or were untreated (bar 4). (C) Ferrets were treated orally with BCX-1812 at 10 mg/kg (bar 1), BCX-1812 at 30 mg/kg (bar 2), or BCX-1812 at 100 mg/kg (bar 3) or were untreated (bar 4). All treated animals were given compound for 3 days beginning 2 h prior to infection with influenza virus clone 7a. Values are means + standard errors of the means (error bars) for three to four animals.
Effect of oral administration (25 mg/kg) of BCX-1827, BCX-1923, and BCX-1812 (RWJ-270201) on influenza infection in ferrets.
The results obtained with 5-mg/kg doses of oseltamivir in the above experiment were very similar to those observed previously (18), but inhibition of both respiratory virus and inflammation as well as fever was improved by increasing the dose to 25 mg/kg (18). Thus, a further experiment was carried out in which groups of four ferrets received oral 25-mg/kg doses of BCX-1827 twice daily as described above, and the outcome with this compound was compared with those with two other cyclopentane derivatives, BCX-1923 and BCX-1812.
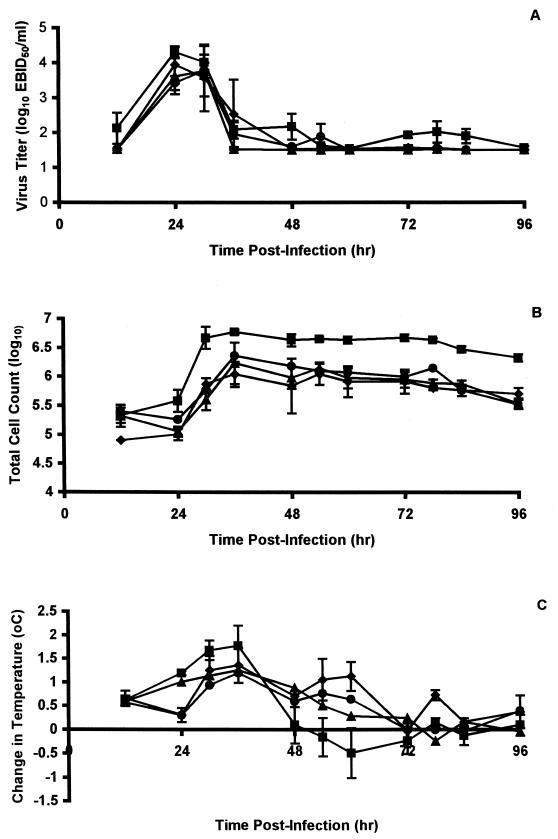
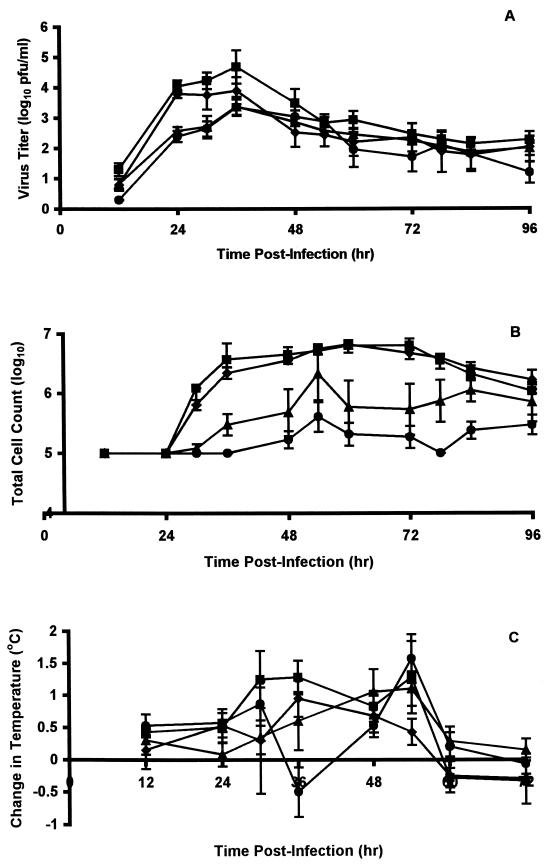
None of the compounds showed significant reductions in virus replication in the upper respiratory tract of ferrets, although virus titers in nasal washes of animals treated with BCX-1812 were slightly delayed and significantly lower 24 hpi (P < 0.01), and there was little evidence of the second peak of replication observed in the untreated control animals (Fig. 4A). However, nasal inflammatory cell counts were delayed in reaching peak levels, which were reduced (three- to fivefold) by all three compounds and remained lower until 96 hpi (Fig. 4B). Similarly, febrile responses were delayed and reduced (Fig. 4C). Virus titers in lung were reduced in animals treated with BCX-1812 and BCX-1923, but not BCX-1827, although they were not reduced significantly (Fig. 3B).
FIG. 4.
Effect of oral administration of compounds on nasal virus titers, nasal inflammatory cell counts, and fever of ferrets infected with influenza virus A (H3N2). Nasal virus titers (A), total numbers of inflammatory cells in nasal washes (B), and changes in temperature relative to baseline temperatures (C) were monitored in ferrets at the indicated times after infection. Ferrets were left untreated (▪) or were treated twice daily with 25-mg/kg oral doses of BCX-1827 (⧫), BCX-1823 (▴), or BCX-1812 (•) for 3 days beginning 2 h prior to infection with influenza virus clone 7a. Values are means ± standard errors of the means (error bars) for three to four animals.
Effects of oral administration with different doses of BCX-1812 (RWJ-270201) on influenza infection in ferrets.
The above results suggested that oral dosing of ferrets with compounds had a markedly suppressive effect on sickness in ferrets without affecting replication to any significant extent in either the upper or lower respiratory tract. To investigate whether this was largely an effect of dosage, BCX-1812 treatment was compared at doses of 10, 30, and 100 mg/kg, the dosing schedule being the same as that described above.
While the 10-mg/kg dose had little effect on virus replication at any one time point in the upper respiratory tract, measurement of the areas under the curve (57.36 ± 10.7 arbitrary units) showed a reduction, although not statistically significant (P > 0.05), in overall virus shedding compared to that observed in untreated animals (74.11 ± 7.83 arbitrary units) (values are means ± standard deviations [SD]). Both the 30- and 100-mg/kg doses, however, had a marked effect. Animals treated with doses of 30 and 100 mg/kg showed significantly reduced levels of virus in nasal washes at both 24 (P < 0.002) and 30 (P < 0.02) hpi, with a further significant (P < 0.05) reduction (20-fold) in peak titers at 36 hpi (Fig. 5A). There was little difference between the responses to these two doses as indicated by measurement of areas under the curve, which were, however, significantly (P < 0.02) smaller (51.8 ± 2.90 and 45.03 ± 7.93 arbitrary units for doses of 30 and 100 mg/kg, respectively) than in untreated animals and animals treated (74.11 ± 7.83 arbitrary units) with the 10-mg/kg dose of BCX-1812.
FIG. 5.
Effect of oral administration of compounds on nasal virus titers, nasal inflammatory cell counts, and fever of ferrets infected with influenza virus A (H3N2). Nasal virus titers (A), total numbers of inflammatory cells in nasal washes (B), and changes in temperature relative to baseline temperatures (C) were monitored in ferrets at the indicated times after infection. Ferrets were left untreated (▪) or were treated twice daily with oral doses of BCX-1812 at 10 mg/kg (⧫), 30 mg/kg (▴), or 100 mg/kg (•) for 3 days beginning 2 h prior to infection with influenza virus clone 7a. Values are means ± standard errors of the means (error bars) for three to four animals.
The inflammatory response in animals treated with the 10-mg/kg dose of BCX-1812 was similar to that in untreated control animals, but the response in animals treated with the 30-mg/kg dose was considerably decreased (12-fold) and the peak was significantly (P < 0.05) reduced (Fig. 5B). The reduction observed with the 100-mg/kg dose was even more marked (Fig. 5B). As measured by areas under the curve, the overall nasal inflammatory responses in animals treated with the 30-mg/kg (27.92 ± 9.99 arbitrary units) or the 100-mg/kg (9.33 ± 2.27 arbitrary units) dose were significantly lower (P < 0.03 and P < 0.001, respectively) than that in untreated animals (54.93 ± 3.16 arbitrary units).
Animals treated with BCX-1812 at 10 mg/kg showed little fever, although some individuals showed more-prolonged low-level fevers, suggesting that the antiviral was not as effective in all animals at this dose (Fig. 5C). The fevers in animals given 30 and 100 mg/kg were delayed and were of short duration, but results were variable, as some animals exhibited little or no fever (Fig. 5C). Nevertheless, calculations of areas under the curve showed that the overall level of fever in animals treated with BCX-1812 at 100 mg/kg (3.16 ± 0.67 arbitrary units) was significantly less than that in control untreated animals (7.85 ± 1.89 arbitrary units). The areas under the curve for animals treated with doses of 10 mg/kg (4.59 ± 1.39) and 30 mg/kg (5.57 ± 2.10), while reduced, were not statistically different from untreated animals.
All doses of compound produced some reduction in virus titers in lung (Fig. 3C). In animals treated with 10 and 30 mg/kg, mean titers were reduced approximately 10-fold, but largely due to a significant reduction in only one of the four animals in each group. In contrast, mean lung titers were reduced approximately 60-fold in animals treated with 100-mg/kg doses of compound, but again this was not statistically significant (P > 0.05).
BCX-1812 (RWJ-270201) pharmacokinetics.
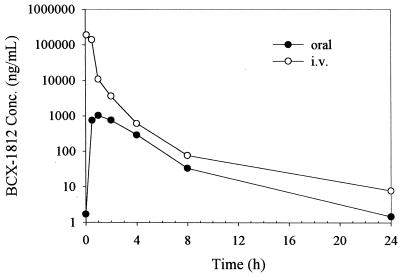
Profiles of mean concentration in plasma versus time are presented in Fig. 6. BCX-1812 was rapidly absorbed following an oral dose of 10 mg/kg to male ferrets based on a mean time to maximum concentration of drug in serum (time to Cmax [Tmax]) of 1.00 h, and was moderately eliminated following both the oral and i.v. doses based on mean half-lives of 3.20 and 3.65 h, respectively. The mean absolute bioavailability following a single oral dose of BCX-1812 was low at 2.5% in male ferrets. The mean volume of distribution following the i.v. dose (10 mg/kg) was 355 ml/kg, which approximates total body water, indicating that the drug was uniformly distributed throughout the body. A summary of the pharmacokinetic parameters (means and SD) for BCX-1812 following oral and i.v. administration at 10 mg/kg to male ferrets is shown in Table 1.
FIG. 6.
Profiles of mean (n = 3) concentration of BCX-1812 plasma in male ferrets following single oral and i.v. administration of BCX-1812 (10 mg/kg).
TABLE 1.
Summary of pharmacokinetic parameters for RWJ-270201 in plasmaa
| Route | Mean (SD) for indicated parameterb
|
||||||
|---|---|---|---|---|---|---|---|
| Cmaxc (ng/ml) | Tmax (h) | AUC0-∞ (ng · h/ml) | t1/2 (h) | CL (ml/h · kg) | Vss (ml/kg) | F (%) | |
| Oral | 1,024 (1,092) | 1.00 (0) | 3,402 (3,790) | 3.20 (1.63) | 5,964 (4,121) | NAd | 2.5 (2.8) |
| i.v. | 202,149 (316,433) | NA | 135,553 (194,284) | 3.65 (0.32) | 298 (239) | 355 (306) | NA |
Parameters were determined using groups of three male ferrets following administration of a single oral or i.v. dose of 10 mg/kg.
Abbreviations for parameters: AUC0-∞, area under the concentration-time curve from 0 h to infinity; t1/2, half-life; CL, clearance; Vss, volume of distribution at steady state; F, bioavailability.
Cmax was calculated as C0 for i.v. dose.
NA, not applicable.
DISCUSSION
A number of antineuraminidase compounds have now reached the clinic, including zanamivir and oseltamivir (36). Zanamivir is a potent antiviral, but it has the disadvantage of being poorly bioavailable in both ferrets and humans (35) and has to be administered intranasally or by aerosol. However, resistant mutants have not been observed in immunocompetent persons treated clinically with zanamivir (5, 7). In contrast, oseltamivir is more bioavailable and can be taken orally as its prodrug, but mutants arise with some frequency in the clinic (32).
A novel series of cyclopentane derivatives, which are structurally different from zanamivir and oseltamivir, have been shown to be inhibitory against influenza virus types A and B in tissue culture and neuraminidase enzyme assays. The 50% effective concentrations and IC50s of these compounds are comparable to those of zanamivir and oseltamivir (4, 25). Several of these, including BCX-1812 (RWJ-270201), were found to be orally active in mice, preventing death, inhibiting lung consolidation and reducing virus titers in the lung (24). RWJ-270201 appears to have a similar pharmacokinetic profile to oseltamivir in mice (24).
While the mouse has usually been the animal of choice for studies of antiviral compounds against influenza virus because of its low cost, ready availability, and ease of handling (22), most influenza viruses, particularly if they are clinical isolates not previously used in mice, cause only a mild subclinical infection of the upper and lower respiratory tracts (27). Following mouse passage (or adaptation) virus strains cause markedly more lung involvement, allowing assessment of disease by time to death, lung weight at death, and histopathology (22). However, influenza in humans is mainly an upper respiratory tract infection with respiratory (nasal discharge and cough) and constitutional (fever, myalgia, and malaise) symptoms but rare pneumonia, except in the elderly, where it mostly results from bacterial superinfection (26). Since ferrets can be infected with human clinical isolates directly without passage and produce both respiratory and constitutional symptoms, which can be quantified, they are perhaps a more predictive and objective model than mice for uncomplicated influenza infections (19, 27).
Of the three cyclopentane compounds tested in this study in ferrets, RWJ-270201 was the most effective in eliminating all respiratory and constitutional signs of infection, including the febrile response, especially at the highest dose of 100 mg/kg. This dose also produced significant reductions in nasal virus yields and virus titers in lung, although in the latter case these reductions were not statistically significant. The bioavailability of RWJ-270201, like that of oseltamivir, appears relatively poor in ferrets. While bioavailability studies with RWJ-270201 have not yet been reported for other species, ferrets showed the lowest oseltamivir bioavailability (11%) in comparison with dogs, rats, and mice (30 to 73%) (16). RWJ-270201 was absorbed more quickly than oseltamivir, with a Tmax of 1 h and a Cmax of 1.02 μg/ml, compared to 2 h and 0.20 μg/ml for oseltamivir, although the dose used for oseltamivir was 5 mg/kg, not 10 mg/kg (16). Consequently levels dropped more quickly but were still above the IC50 for most viruses 24 h postdosing.
These experiments in ferrets have confirmed previous indications of oral efficacy in mice (24) and, taken together with the low toxicity of this compound and hence a high in vivo margin for safety (24), the present results support the choice of RWJ-270201 for further clinical development for treatment of influenza virus infections in humans.
REFERENCES
- 1.Appleyard, G., and H. B. Maber. 1974. Plaque formation by influenza viruses in the presence of trypsin. J. Gen. Virol. 25:351-357. [DOI] [PubMed] [Google Scholar]
- 2.Atigadda, V. R., W. J. Brouillette, F. Duarte, S. M. Ali, Y. S. Babu, S. Bantia, P. Chand, N. Chu, J. A. Montgomery, D. A. Walsh, E. A. Sudbeck, J. Finley, M. Luo, G. M. Air, and G. W. Laver. 1999. Potent inhibition of influenza sialidase by a benzoic acid containing a 2-pyrrolidinone substituent. J. Med. Chem. 42:2332-2343. [DOI] [PubMed] [Google Scholar]
- 3.Babu, Y. S., P. Chand, S. Bantia, P. L. Kotian, A. Dehghani, Y. El-Kattan, T. Lin, T. L. Hutchinson, A. J. Elliott, C. D. Parker, S. L. Ananth, L. L. Horn, G. W. Laver, and J. A. Montgomery. 2000. BCX-1812 (RWJ-270201): discovery of a novel, highly potent, orally active, and selective influenza neuraminidase inhibitor through structure-based drug design. J. Med. Chem. 43:3482-3486. [DOI] [PubMed] [Google Scholar]
- 4.Bantia, S., C. D. Parker, S. L. Ananth, L. L. Horn, K. Andries, P. Chand, P. L. Kotian, A. Dehghani, Y. El-Kattan, T. Lin, T. L. Hutchinson, J. A. Montgomery, D. L. Kellog, and Y. S. Babu. 2001. Comparison of anti-influenza virus activity of RWJ-270201 with those of oseltamivir and zanamivir. Antimicrob. Agents Chemother. 45:1162-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnett, J. M., A. Cadman, D. Gor, M. Dempsey, M. Walters, A. Candlin, M. Tisdale, P. J. Morley, I. J. Owens, R. J. Fenton, A. P. Lewis, E. C. J. Claas, G. F. Rimmelzwaan, R. De Groot, and A. D. M. E. Osterhaus. 2000. Zanamivir susceptibility monitoring and characterization of influenza virus clinical isolates obtained during phase II clinical efficacy studies. Antimicrob. Agents Chemother. 44:78-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bean, W. J., S. C. Threlkeld, and R. G. Webster. 1989. Biologic potential of amantadine-resistant influenza A virus in an avian model. J. Infect. Dis. 159:1050-1056. [DOI] [PubMed] [Google Scholar]
- 7.Boivin, G., N. Goyette, I. Hardy, F. Y. Aoki, A. Wagner, and S. Trottier. 2000. Rapid antiviral effect of inhaled zanamivir in the treatment of naturally occurring influenza in otherwise healthy adults. J. Infect. Dis. 181:1471-1474. [DOI] [PubMed] [Google Scholar]
- 8.Burmeister, W. P., R. W. Ruigrok, and S. Cusack. 1992. The 2.2A resolution crystal structure of influenza B neuraminidase and its complex with sialic acid. EMBO J. 11:49-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell, D., C. Sweet, and H. Smith. 1979. Comparisons of virulence of influenza virus recombinants in ferrets in relation to their behaviour in man and their genetic constitution. J. Gen. Virol. 44:37-44. [DOI] [PubMed] [Google Scholar]
- 10.Colacino, J. M., D. C. Delong, J. R. Nelson, W. A. Spitzer, J. Tang, F. Victor, and C. Y. E. Wu. 1990. Evaluation of the anti-influenza virus activities of 1,3,4-thiadiazol-2-ylcyanamide (LY217896) and its sodium salt. Antimicrob. Agents Chemother. 34:2156-2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies, W. L., R. F. Grunert, J. W. Haff, J. W. McGahen, E. M. Neumayer, M. Paulshock, J. C. Watts, T. R. Wood, E. C. Hermann, and C. E. Hoffman. 1964. Antiviral activity of 1-adamantanamine (amantadine). Science 144:862-864. [DOI] [PubMed] [Google Scholar]
- 12.Hay, A. J., M. C. Zambon, A. J. Wolstenholme, J. J. Skehel, and M. H. Smith. 1986. Molecular basis of resistance of influenza A viruses to amantadine. J. Antimicrob. Chemother. 18(Suppl. B):19-29. [DOI] [PubMed] [Google Scholar]
- 13.Hayden, F. G., S. J. Sperber, R. B. Belshe, R. D. Clover, A. J. Hay, and S. Pyke. 1991. Recovery of drug-resistant influenza A virus during therapeutic use of rimantadine. Antimicrob. Agents Chemother. 35:1741-1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayden, F. G., J. J. Treanor, A. F. Betts, M. Lobo, J. D. Esinhart, and E. K. Hussey. 1996. Safety and efficacy of the neuraminidase inhibitor GG167 in experimental human influenza. JAMA 275:295-299. [PubMed] [Google Scholar]
- 15.Kim, C. U., W. Lew, M. A. Williams, H. Liu, L. Zhang, S. Swaminathan, N. Bischofberger, M. S. Chen, D. B. Mendel, C. Y. Tai, W. G. Laver, and R. C. Stevens. 1997. Influenza neuraminidase inhibitors possessing a novel hydrophobic interaction in the enzyme active site: design, synthesis, and structural analysis of carbocyclic sialic acid analogues with potent anti-influenza activity. J. Am. Chem. Soc. 119:681-690. [DOI] [PubMed] [Google Scholar]
- 16.Li, W., P. A. Escarpe, E. J. Eisenberg, K. C. Cundy, C. Sweet, K. J. Jakeman, J. Merson, W. Lew, M. Williams, L. Zhang, C. U. Kim, N. Bischofberger, M. S. Chen, and D. B. Mendel. 1998. Identification of GS4104 as an orally bioavailable prodrug of the influenza virus neuraminidase inhibitor GS4071. Antimicrob. Agents Chemother. 42:647-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuyama, T., C. Sweet, M. H. Collie, and H. Smith. 1980. Aspects of virulence in ferrets exhibited by influenza virus recombinants of known genetic composition. J. Infect. Dis. 141:351-361. [DOI] [PubMed] [Google Scholar]
- 18.Mendel, D. B., C. Y. Tai, P. A. Escarpe, W. Li, R. W. Sidwell, J. H. Huffman, C. Sweet, K. J. Jakeman, J. Merson, S. A. Lacy, W. Lew, M. A. Williams, L. Zhang, M. S. Chen, N. Bischofberger, and C. U. Kim. 1998. Oral administration of a prodrug of the influenza virus neuraminidase inhibitor GS4071 protects mice and ferrets against influenza infection. Antimicrob. Agents Chemother. 42:640-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Renegar, K. B. 1992. Influenza virus infections and immunity: a review of human and animal models. Lab. Anim. Sci. 42:222-232. [PubMed] [Google Scholar]
- 20.Reuman, P. D., S. Keely, and G. M. Schiff. 1989. Assessment of signs of influenza illness in the ferret model. J. Virol. Methods 24:27-34. [DOI] [PubMed] [Google Scholar]
- 21.Ryan, D. M., J. Ticehurst, M. H. Dempsey, and C. R. Penn. 1994. Inhibition of influenza virus replication in mice by GG167 (4-guanidino-2,4-dideoxy-2,3-dehydro-N-acetylneuraminic acid) is consistent with extracellular activity of viral neuraminidase (sialidase). Antimicrob. Agents Chemother. 38:2270-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sidwell, R. W. 1999. The mouse model of influenza virus infection, p. 981-987. In O. Zak and M. Sande (ed.), Handbook of animal models of infection. Experimental models of antimicrobial chemotherapy. Academic Press, London, United Kingdom.
- 23.Sidwell, R. W., J. H. Huffman, G. P. Khare, L. B. Allen, J. T. Witkowski, and R. K. Robins. 1972. Blood-stream antiviral activity of virazole: 1-β-D-ribofuranosyl-1,2,4-triazole-3-carboxamide. Science 177:705-706. [DOI] [PubMed] [Google Scholar]
- 24.Sidwell, R. W., D. F. Smee, J. H. Huffman, D. L. Barnard, K. W. Bailey, J. D. Morrey, and Y. S. Babu. 2001. In vivo influenza virus-inhibitory effects of the cyclopentane neuraminidase inhibitor RWJ-270201. Antimicrob. Agents Chemother. 45:749-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smee, D. F., J. H. Huffman, A. C. Morrison, D. L. Barnard, and R. W. Sidwell. 2001. Cyclopentane neuraminidase inhibitors with potent in vitro anti-influenza virus activities. Antimicrob. Agents Chemother. 45:743-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith, H., and C. Sweet. 1988. Lessons for human influenza from pathogenicity studies with ferrets. Rev. Infect. Dis. 10:56-75. [DOI] [PubMed] [Google Scholar]
- 27.Sweet, C., R. J. Fenton, and G. E. Price. 1999. The ferret as an animal model of influenza virus infection, p. 989-998. In O. Zak and M. Sande (ed.), Handbook of animal models of infection. Experimental models of antimicrobial chemotherapy. Academic Press, London, United Kingdom.
- 28.Sweet, C., F. G. Hayden, K. J. Jakeman, S. Grambas, and A. J. Hay. 1991. Virulence of rimantidine-resistant human influenza A (H3N2) viruses in ferrets. J. Infect. Dis. 162:969-972. [DOI] [PubMed] [Google Scholar]
- 29.Sweet, C., J. Stephen, and H. Smith. 1974. Purification of influenza viruses using disulphide-linked immunosorbents derived from rabbit antibody. Immunochemistry 11:295-304. [DOI] [PubMed] [Google Scholar]
- 30.Toms, G. L., R. A. Bird, S. M. Kingsman, C. Sweet, and H. Smith. 1976. The behaviour in ferrets of two closely related clones of influenza virus of differing virulence for man. Br. J. Exp. Pathol. 57:37-48. [PMC free article] [PubMed] [Google Scholar]
- 31.Toms, G. L., J. A. Davies, C. G. Woodward, C. Sweet, and H. Smith. 1977. The relation of pyrexia and nasal inflammatory response to virus levels in nasal washings of ferrets infected with influenza viruses of differing virulence. Br. J. Exp. Pathol. 58:444-458. [PMC free article] [PubMed] [Google Scholar]
- 32.Treanor, J. J., F. G. Hayden, P. S. Vrooman, R. A. Barbarash, R. Bettis, D. Riff, S. Singh, N. Kinnersley, P. Ward, and R. G. Mills. 2000. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. JAMA 283:1016-1024. [DOI] [PubMed] [Google Scholar]
- 33.Tuttle, J. V., M. Tisdale, and T. A. Krenitsky. 1993. Purine 2′-deoxy-2′fluororibosides as anti-influenza virus agents. J. Med. Chem. 36:119-125. [DOI] [PubMed] [Google Scholar]
- 34.Varghese, J. N., J. L. McKimm-Breschkin, J. B. Caldwell, A. A. Kortt, and P. M. Colman. 1992. The structure of the complex between influenza virus neuraminidase and sialic acid, the viral receptor. Proteins 14:327-332. [DOI] [PubMed] [Google Scholar]
- 35.von Itzstein, M., W.-Y. Wu, G. B. Kok, M. S. Pegg, J. C. Dyason, B. Jin, T. V. Phan, M. L. Smythe, H. F. White, S. W. Oliver, P. M. Colman, J. N. Varghese, D. M. Ryan, J. M. Woods, R. C. Bethell, V. J. Hotham, J. M. Cameron, and C. R. Penn. 1993. Rational design of potent sialidase-based inhibitors of influenza virus replication. Nature 363:418-423. [DOI] [PubMed] [Google Scholar]
- 36.Zambon, M., and F. G. Hayden. 2001. Position statement: global neuraminidase inhibitor susceptibility network. Antivir. Res. 49:147-156. [DOI] [PubMed] [Google Scholar]