Abstract
Tenofovir is a nucleotide analogue human immunodeficiency virus type 1 (HIV-1) reverse transcriptase (RT) inhibitor, and its oral prodrug, tenofovir disoproxil fumarate, has recently been approved for the treatment of HIV-1 infection in the United States. The objective of this study was to characterize the in vitro susceptibility profiles of a large panel of clinically derived HIV-1 isolates for tenofovir. The distribution of tenofovir susceptibilities in over 1,000 antiretroviral-naive, HIV-1-infected individuals worldwide was determined using the Virco Antivirogram assay. In addition, phenotypic susceptibilities to tenofovir and other RT inhibitors were determined in a panel of nearly 5,000 recombinant HIV-1 clinical isolates from predominantly treatment-experienced patients analyzed as a part of routine drug resistance testing. Greater than 97.5% of isolates from treatment-naive patients had tenofovir susceptibilities <3-fold above those of the wild-type controls by the Antivirogram. The clinically derived panel of 5,000 samples exhibited a broad range of antiretroviral drug susceptibilities, including 69, 43, and 16% having >10-fold-decreased susceptibilities to at least one, two, and three antiretroviral drug classes, respectively. Greater than 88% of these 5,000 clinical isolates were within the threefold susceptibility range for tenofovir, and >99% exhibited <10-fold-reduced susceptibilities to tenofovir. Decreased susceptibility to tenofovir was not directly associated with resistance to other RT inhibitors; r2 values of log-log linear regression plots of susceptibility to tenofovir versus susceptibility to other RT inhibitors were <0.4. The results suggest that the majority of treatment-naive and treatment-experienced individuals harbor HIV that remains within the normal range of tenofovir susceptibilities and may be susceptible to tenofovir disoproxil fumarate therapy.
Tenofovir [R-9-(2-phosphonomethoxypropyl)adenine (PMPA)]is a nucleotide analogue inhibitor of the human immunodeficiency virus type 1 (HIV-1) reverse transcriptase (RT). Tenofovir and its oral prodrug, tenofovir disoproxil fumarate (tenofovir DF), have been investigated previously in vitro (7, 9, 13, 14), in vivo in animal models (8, 10, 15-19), and in clinical trials with human subjects (1, 2). Tenofovir DF has recently been approved for clinical use in the treatment of HIV-1 infection in the United States.
To our knowledge, there are no published studies investigating the natural distribution of tenofovir drug susceptibilities among treatment-naive individuals. Similarly, there have been very few published studies relating to mutations in the HIV-1 RT that may potentially confer resistance to tenofovir among treatment-experienced individuals. It has been previously shown in vitro (13, 20) and in vivo in simian immunodeficiency virus (SIV)-infected rhesus macaques (17) that the K65R mutation in the HIV and SIV RT confers three- to fivefold-decreased susceptibilities to tenofovir. This mutation has also been selected at low frequency in vivo in tenofovir DF-treated patients (M. D. Miller et al., 5th Int. Conf. Drug Ther. HIV Infect., abstr. 326, 2001). With respect to cross-resistance, several small studies have reported that, in general, the cross-resistance profile for tenofovir appears to be limited (13, 20; M. D. Miller et al., 4th Int. Workshop HIV Drug Resist. Treatment Strategies, abstr. 4, 2000), although more definitive research is needed on this subject.
The focus of this study was to determine the natural range of tenofovir phenotypic susceptibilities in vitro of clinically derived HIV-1 recombinant isolates from over 1,000 antiretroviral-naive individuals participating in seven clinical studies in the United States, Germany, Canada, and South Africa and to use this information to establish a biologically relevant value for interpreting phenotypic antiretroviral susceptibility data. In addition, we characterized the profile of tenofovir cross-resistance in nearly 5,000 clinically derived recombinant isolates, including many that were highly nucleoside resistant, and identified potential mutations in the HIV RT that confer large decreases in tenofovir susceptibility.
MATERIALS AND METHODS
Study design and patients.
The characteristics and origin of the approximately 1,000 antiretroviral-naive plasma samples utilized in this study are described in a previous report (4). The plasma samples from treatment-experienced patients (n ∼ 5,000) corresponded to consecutive, routine clinical samples sent to Virco for genotypic and phenotypic analysis from the United States and Europe from July to December 2000 (3). Because they were sent for resistance testing, these samples mostly represent treatment-experienced individuals failing their current regimen. However, treatment history is generally unknown, and the samples may not represent a random cross section of the entire HIV-1-infected population.
HIV RNA extraction and genotyping.
Viral RNA was extracted from 200-μl samples of patient plasma with the QIAamp viral RNA extraction kit (Qiagen, Hilden, Germany), according to the manufacturer's instructions. DNA encompassing part of the pol gene was produced using Expand Reverse Transcriptase (Boehringer Mannheim) as described previously (5). A 2.2-kb fragment encoding the protease and RT regions was then amplified by nested PCR and sequenced using previously described primers and conditions (5). The results of the genotypic analysis were reported as amino acid changes at positions in the RT gene compared to the sequence of wild-type reference strain HXB2.
Phenotypic testing.
Phenotypic drug susceptibility analysis was performed by a recombinant assay (5) (Virco, Mechelen, Belgium) with modifications as described elsewhere (R. Pauwels, K. Hertogs, B. A. Larder, et al., 2nd Int. Workshop HIV Drug Resist. Treatment Strategies, abstr. 51, 1998). Briefly, protease- and RT-encoding sequences were amplified from patient-derived viral RNA with HIV-1-specific primers. After homologous recombination of amplicons into a proviral clone from which the protease- and RT-encoding sequences were deleted, the resulting recombinant viruses were harvested, titrated, and used for testing of in vitro susceptibility to antiviral drugs. The mean IC50 (the amount of drug required to inhibit recombinant viral production by 50%) was then compared to the mean IC50 of the same drug obtained when tested with a control laboratory wild-type virus isolate (HIV-1 strain IIIB), and fold change values were reported.
Statistical analysis of cross-resistant isolates.
The observed degree of cross-resistance to tenofovir and other antiretroviral drugs in our panel of treatment-experienced isolates was quantified using a linear regression of the log fold change in susceptibilities to different antiretroviral drugs. The results were expressed in terms of the square of the correlation coefficient, r2.
RESULTS
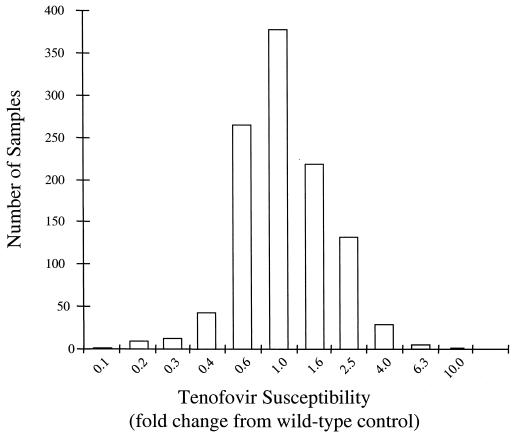
The average phenotypic susceptibility (measured in a recombinant virus assay) to tenofovir was determined for HIV-1 samples from >1,000 treatment-naive, HIV-1-positive individuals from four countries (the United States, Canada, Germany, and South Africa). Phenotypic susceptibility to tenofovir in this population approximated a symmetrical log-normal distribution, with the geometric mean fold change in susceptibility from that of wild type falling near 1.0 (Fig. 1). The upper normal range for tenofovir susceptibility was defined as the geometric mean + 2 standard deviations, a range that included ∼97.5% of the treatment-naive samples. This upper limit corresponded to a threefold increase in IC50 with respect to the wild-type control.
FIG. 1.
Tenofovir susceptibilities of HIV-1 isolates obtained from antiretroviral-naive individuals. The frequency distribution (log scale) of tenofovir susceptibilities in more than 1,000 recombinant isolates obtained from antiretroviral-naive individuals was determined by the Antivirogram recombinant phenotypic assay.
The profile of phenotypic cross-resistance to tenofovir and other classes of antiretroviral drugs (nucleoside analogue RT inhibitors, nonnucleoside RT inhibitors [NNRTIs], and protease inhibitors [PIs]) was assessed using a panel of ∼5,000 consecutive clinical samples submitted to Virco for routine drug resistance testing during the second half of 2000. Because drug resistance tests had been ordered, these samples most likely represented treatment-experienced individuals failing their current antiretroviral regimens. Phenotypic analyses of these samples revealed that 69% of samples had >10-fold-decreased susceptibility to a drug from at least one class, 43% had >10-fold-decreased susceptibility to drugs from at least two classes, and 16% had >10-fold-decreased susceptibility to drugs from all three classes. Within the nucleoside analogue class, the greatest degree of resistance was observed to lamivudine (3TC) and zidovudine (AZT) with 40 and 17% of samples, respectively, having >10-fold-decreased susceptibility compared to that for the wild type. Within the NNRTI class, 49% had >10-fold-decreased susceptibility to at least one NNRTI.
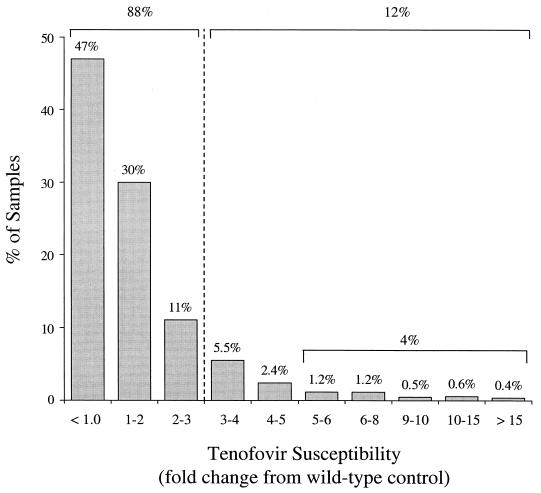
Over 88% of these 5,000 samples were within the threefold normal range of tenofovir susceptibility (Fig. 2). Large decreases in tenofovir susceptibility were quite rare, with only 4% of samples having >5-fold decreases in tenofovir susceptibility and only 1% of samples showing >10-fold decreases in tenofovir susceptibility.
FIG. 2.
Tenofovir susceptibilities of HIV-1 isolates obtained from individuals undergoing routine HIV drug resistance testing. The distribution of tenofovir susceptibilities in a panel of ∼5,000 consecutive recombinant clinical isolates from individuals requesting drug resistance testing was determined by the Antivirogram phenotypic assay.
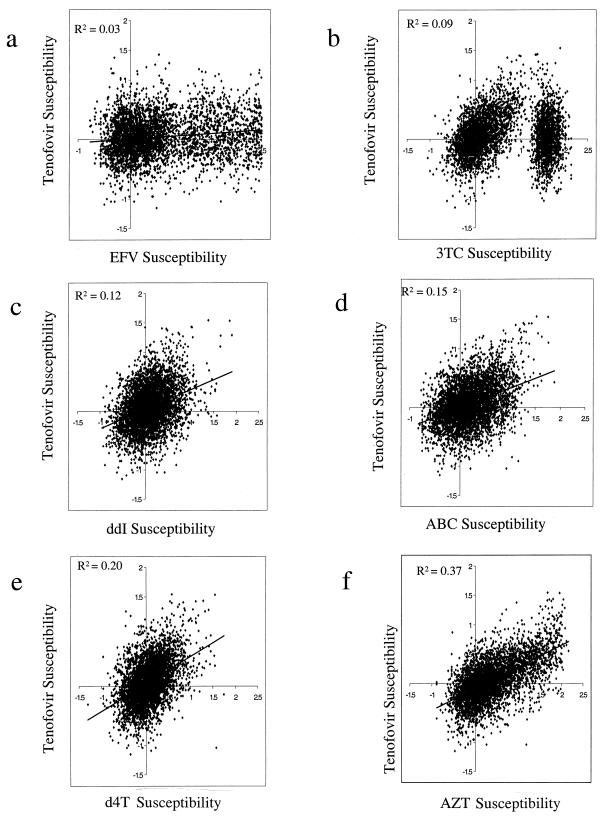
Linear regression analysis of the log fold change in susceptibilities to different antiretroviral drugs was used to quantify the degree of cross-resistance to tenofovir and other antiretroviral drugs in our panel. Susceptibilities to tenofovir and to drugs of the NNRTI class (represented by efavirenz), as well as the nucleoside analogue class (3TC, didanosine, abacavir [ABC], stavudine [d4T], and AZT), are shown in Fig. 3. The lowest (r2 = 0.03) and highest (r2 = 0.37) correlations were observed with efavirenz and AZT, respectively. All other r2 values fell below 0.20. For 3TC, if the highly 3TC-resistant (>15-fold) isolates are excluded from the analysis, there is a greater correlation between 3TC and tenofovir susceptibilities (r2 = 0.23 [data not shown]).
FIG. 3.
Linear regression analyses of cross-resistance profiles of HIV-1 isolates obtained from individuals undergoing routine drug resistance testing. Tenofovir susceptibility as determined using the Antivirogram recombinant phenotypic assay is indicated on a log-log scale as a function of susceptibility to efavirenz (EFV) (a), 3TC (b), didanosine (c), ABC (d), d4T (e), and AZT (f). Note that the regression line is deliberately omitted in panel b, due to the observation of two distinct populations. Also note that, if the highly 3TC-resistant isolates (represented by the population on the right in panel b) are excluded from the analysis, the correlation between 3TC and tenofovir becomes stronger (r2 = 0.23).
The tenofovir susceptibility of HIV-1 samples with reduced susceptibility to nucleoside analogue RT inhibitors was also investigated (Table 1). Samples with reduced susceptibility to AZT, 3TC, d4T, didanosine, and ABC, defined as greater than the biological cutoff for each drug, remained within the threefold normal range of tenofovir susceptibility in 62 to 85% of samples (Table 1). The greatest cross-resistance was observed for samples with reduced susceptibility to d4T, for which only 25 and 40% remained within the susceptible range for AZT and ABC, respectively. These results and those of the linear regression analyses suggest a complicated pattern of cross-resistance to nucleoside and nucleotide analogues where partial cross-resistance is evident and differs among the analogues.
TABLE 1.
Reduced susceptibility of HIV-1 to nucleoside analoguesa
| Fold reduced NRTI susceptibility (n) | % within normal range
|
||||
|---|---|---|---|---|---|
| TDF | ABC | AZT | d4T | ddI | |
| >4 AZT (1,423) | 71 | 48 | NA | 74 | 75 |
| >4.5 3TC (2,185) | 85 | 54 | 59 | 87 | 77 |
| >3 d4T (475) | 62 | 40 | 25 | NA | 55 |
| >3.5 ddI (496) | 72 | 30 | 61 | 65 | NA |
| >3 ABC (1,191) | 76 | NA | 38 | 76 | 71 |
Cutoffs for each drug were set at the biological cutoff of 2 standard deviations from the mean susceptibility value in untreated patients. ddI, dideoxyinosine; TDF, tenofovir DF; NA, not applicable.
The samples which displayed >10-fold-decreased susceptibility to tenofovir (n = 51, or 1%) were examined separately. Among these 51 isolates, resistance to tenofovir was most strongly associated with high-level AZT resistance, with only one sample remaining within the normal range of AZT susceptibility. Of the 51 highly resistant isolates, 43 (84%) had multiple mutations associated with AZT resistance, including the T215Y/F substitution in all cases. The median fold decrease in AZT susceptibility overall in this group was 54-fold. The samples with >10-fold-decreased susceptibility to tenofovir had variable susceptibility to other nucleoside analogues, with 37 and 53% remaining in the normal range for d4T and didanosine, respectively. However, 29% of these 51 isolates showed reduced susceptibility to all drugs in the nucleoside analogue class.
Genotypic correlates of reduced tenofovir susceptibility were also investigated. The K65R substitution, previously associated with tenofovir resistance in vitro (13, 20) and in SIV-infected macaques (17), and the Q151M substitution, associated with multinucleoside resistance in combination with other mutations (11, 12), were only infrequently observed in the study samples. Only 37 of ∼5,000 isolates (<1%) harbored the K65R mutation. Of these, 21 (56%) and 4 (11%) showed decreases in tenofovir susceptibility of >3-fold and >10-fold, respectively. Seventy of ∼5,000 isolates (>1.5%) harbored the Q151M mutation, of which only 10 (14%) and 4 (6%) showed decreases in tenofovir susceptibility of >3-fold and >10-fold, respectively. There was a tendency for nucleoside RT inhibitor (NRTI)-associated mutations (NAMs), including the M41L, D67N, K70R, L210W, T215Y/F, and K219Q substitutions in the HIV RT (typically associated with decreased susceptibility to thymidine analogues), to also be associated with susceptibility to tenofovir. Accumulation of these NAMs was associated with progressive decreases in tenofovir susceptibility. Of the 650 isolates harboring at least one of these NAMs, the observed median fold decreases in tenofovir susceptibility were 1.0, 2.0, and 3.2 for isolates with one or two, three or four, and five or six of these NAMs, respectively. This effect, however, could be partially reversed by the M184V mutation. Of the 720 isolates harboring at least one of these NAMs in combination with the M184V substitution, the observed median fold decreases in tenofovir susceptibility were 0.8, 1.5, and 2.0 for isolates with one or two, three or four, and five or six of these NAMs, respectively.
Finally, 32 of ∼5,000 isolates (<1%) contained the T69S double insertion, which was associated with a >3-fold- or >10-fold-reduced susceptibility to tenofovir in 26 (81%) or 18 (56%) of cases, respectively.
DISCUSSION
We assessed the range of phenotypic susceptibilities to tenofovir in vitro using clinically derived HIV-1 recombinant isolates from over 1,000 drug-naive individuals worldwide and used this information to establish biologically relevant cutoff values for phenotypic antiretroviral susceptibility testing in the Virco Antivirogram assay (4). The threefold cutoff, obtained by adding 2 standard deviations to the geometric mean susceptibility value, included >97.5% of the study population. This value is similar to that obtained using the same samples for determination of the natural range of susceptibilities to d4T and didanosine (4). Note, however, that this value represents a reference for defining a degree of susceptibility which would be expected to be only rarely observed in an untreated population, i.e., an upper limit of normal. Although the clinically significant degree of resistance to tenofovir in vivo is yet to be established, information included in the U.S. package insert for tenofovir DF tablets associates >4-fold baseline resistance to tenofovir with reduced virologic response to tenofovir DF.
In the design and investigation of potential new drugs, it is important to consider whether the new drug will be useful as therapy not only for treatment-naive patients but also for patients who may be failing their current regimen. For this reason, we attempted to characterize the profile of cross-resistance to tenofovir in a clinical panel of nearly 5,000 isolates representing predominantly treatment-experienced individuals, a large proportion of whom may have been failing their current therapy. The observation that reduced susceptibility to tenofovir beyond the normal range was infrequent (88% of samples remained within the normal threefold susceptibility range, and 99% fell below 10-fold-reduced susceptibility) among these drug-experienced individuals suggests that a majority of treatment-experienced individuals may retain sensitivity to tenofovir. An investigation into the patterns of cross-resistance to tenofovir and other licensed RT inhibitors revealed the greatest correlation of in vitro resistance pattern to be with AZT (r2 = 0.37), with all other coefficients falling below r2 = 0.20. These values are considerably lower than those previously reported for the members of the PI class to which there is high cross-resistance (where comparisons of log-log susceptibilities to PIs yielded r2 values between 0.63 and 0.79) but are comparable to previously observed levels of cross-resistance to the other members of the nucleoside analogue class (with r2 values ranging from 0.03 for 3TC-d4T to 0.29 for AZT-d4T to 0.42 for 3TC-ABC) (3). An alternative analysis revealed that >3-fold-decreased susceptibility to d4T was most closely associated with decreased tenofovir susceptibility, with 38% of samples also having reduced susceptibility to tenofovir. It is important to note that the samples with reduced d4T susceptibility correspond to the fewest, most highly nucleoside analogue-resistant isolates (n = 475 for d4T versus >1,400 for AZT and 3TC). Overall, these results suggest that tenofovir may retain some antiviral activity even in treatment-experienced individuals with some NRTI-associated resistance mutations, a fact that is consistent with the results of the tenofovir DF phase III clinical trials (K. Squires, G. Pierone, D. Berger, C. Steinhart, N. Bellos, S. Becker, J. Salzer, D. Coakley, S. S. Chen, M. Miller, and A. Cheng for the Study 907 Team, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. I-666, 2001).
Previous studies have investigated the issue of in vitro genotypic markers for tenofovir resistance (6, 20). The observation that the accumulation of common NRTI-associated resistance mutations is associated with decreased tenofovir susceptibility but that this effect decreases with the appearance of the 3TC-associated M184V mutation is consistent with previously reported results from clinical isolates and isolates derived from site-directed mutagenesis (6, 20). The fact that the K65R substitution is only rarely observed in vivo is also consistent with previous surveys (S. Bloor et al., 4th Int. Workshop HIV Drug Resist. Treatment Strategies, abstr. 169, 2000). To date, very few patients treated with tenofovir DF have developed the K65R mutation (Miller et al., 5th Int. Conf. Drug Ther. HIV Infect.). In our panel of ∼5,000 samples, phenotypic-genotypic analyses revealed that high-level (>10-fold) decreased susceptibility to tenofovir was most strongly associated with the HIV-1 RT T69S double insertion and/or multiple resistance mutations associated with AZT resistance, including the T215Y/F mutation. However, this does not necessarily mean that these mutations will be selected by tenofovir therapy in vivo. Studies are under way to characterize those mutations and mutation patterns that may occur as a result of tenofovir DF selection in vivo.
This study evaluated the natural range of susceptibilities and profiles of cross-resistance to tenofovir DF, an anti-HIV nucleotide analogue, in a large, diverse population of HIV-1-infected individuals worldwide. The results from this study suggest that a majority of treatment-naive and treatment-experienced individuals have HIV-1 that may be susceptible to therapy that includes tenofovir DF.
REFERENCES
- 1.Barditch-Crovo, P., S. G. Deeks, A. Collier, S. Safrin, D. F. Coakley, M. Miller, B. P. Kearney, R. L. Coleman, P. D. Lamy, J. O. Kahn, I. McGowan, and P. S. Lietman. 2001. Phase I/II trial of the pharmacokinetics, safety, and antiretroviral activity of tenofovir disoproxil fumarate in human immunodeficiency virus-infected adults. Antimicrob. Agents Chemother. 45:2733-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deeks, S. G., P. Barditch-Crovo, P. S. Lietman, F. Hwang, K. C. Cundy, J. F. Rooney, N. S. Hellmann, S. Safrin, and J. O. Kahn. 1998. Safety, pharmacokinetics, and antiretroviral activity of intravenous 9-[2-(R)-(phosphonomethoxy)propyl]adenine, a novel anti-human immunodeficiency virus (HIV) therapy, in HIV-infected adults. Antimicrob. Agents Chemother. 42:2380-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrigan, P. R., and B. A. Larder. 2002. Extent of cross-resistance between agents used to treat human immunodeficiency virus type 1 infection in clinically derived isolates. Antimicrob. Agents Chemother. 46:909-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harrigan, P. R., J. S. G. Montaner, S. A. Wegner, W. Verbiest, V. Miller, R. Wood, and B. A. Larder. 2001. Worldwide variation in HIV-1 phenotypic susceptibility in untreated individuals: biologically relevant values for resistance testing. AIDS 15:1671-1677. [DOI] [PubMed] [Google Scholar]
- 5.Hertogs, K., M.-P. de Bethune, V. Miller, T. Ivens, P. Schel, A. Van Cauwenberge, C. Van Den Eynde, V. Van Gerwen, H. Azijn, M. Van Houtte, F. Peeters, S. Staszewski, M. Conant, S. Bloor, S. Kemp, B. Larder, and R. Pauwels. 1998. A rapid method for simultaneous detection of phenotypic resistance to inhibitors of protease and reverse transcriptase in recombinant human immunodeficiency virus type 1 isolates from patients treated with antiretroviral drugs. Antimicrob. Agents Chemother. 42:269-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller, M. D., N. A. Margot, K. Hertogs, B. Larder, and V. Miller. 2001. Antiviral activity of tenofovir (PMPA) against nucleoside-resistant clinical HIV samples. Nucleosides Nucleotides Nucleic Acids 20:1025-1028. [DOI] [PubMed] [Google Scholar]
- 7.Mulato, A. S., and J. M. Cherrington. 1997. Anti-HIV activity of adefovir (PMEA) and PMPA in combination with antiretroviral compounds: in vitro analyses. Antivir. Res. 36:91-97. [DOI] [PubMed] [Google Scholar]
- 8.Naesens, L., N. Bischofberger, P. Augustijns, P. Annaert, G. Van Den Mooter, M. N. Arimilli, C. U. Kim, and E. De Clerq. 1998. Antiretroviral efficacy and pharmacokinetics of oral bis(isopropyloxycarbonyloxymethyl)-9-(2-phosphonylmethoxypropyl)adenine in mice. Antimicrob. Agents Chemother. 42:1568-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robbins, B. L., R. Srinivas, C. Kim, N. Bischofberger, and A. Fridland. 1998. Anti-human immunodeficiency virus activity and cellular metabolism of a potential prodrug of the acyclic nucleoside phosphonate 9-R-(2-phosphonomethoxypropyl)adenine (PMPA), bis(isopropyloxymethylcarbonyl)PMPA. Antimicrob. Agents Chemother. 42:612-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenwirth, B., P. Ten Haaft, W. M. J. M. Bogers, I. G. Nieuwenhuis, H. Niphuis, E.-M. Kuhn, N. Bischofberger, J. L. Heeney, and K. Uberla. 2000. Antiretroviral therapy during primary immunodeficiency virus infection can induce persistent suppression of virus load and protection from heterologous challenge in rhesus macaques. J. Virol. 74:1704-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmit, J.-C., K. Van Laethem, L. Ruiz, P. Hermans, S. Sprecher, A. Sonnerborg, M. Leal, T. Harrer, B. Clotet, V. Arendt, E. Lissen, M. Witvrouw, J. Desmyter, E. De Clercq, and A.-M. Vandamme. 1998. Multiple dideoxynucleoside analogue-resistant (MddNR) HIV-1 strains isolated from patients from different European countries. AIDS 12:2007-2015. [DOI] [PubMed] [Google Scholar]
- 12.Shirasaka, T., M. F. Kavlick, T. Ueno, W. Y. Gao, E. Kojima, M. L. Alcaide, S. Chokekijchai, B. M. Roy, E. Arnold, R. Yarchoan, and H. Mitsuya. 1995. Emergence of human immunodeficiency virus type 1 variants with resistance to multiple dideoxynucleosides in patients receiving therapy with dideoxynucleosides. Proc. Natl. Acad. Sci. USA 92:2398-2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Srinivas, R., and A. Fridland. 1998. Antiviral activities of 9-R-2-phosphonomethoxypropyl adenine (PMPA) and bis(isopropyloxymethylcarbonyl)PMPA against various drug-resistant human immunodeficiency virus strains. Antimicrob. Agents Chemother. 42:1484-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suo, Z., and K. A. Johnson. 1998. Selective inhibition of HIV-1 reverse transcriptase by an antiviral inhibitor, (R)-9-(2-phosphonomethoxypropyl)adenine. J. Biol. Chem. 273:27250-27258. [DOI] [PubMed] [Google Scholar]
- 15.Tsai, C.-C., K. Follis, A. Sabo, T. Beck, R. Grant, N. Bischofberger, R. Benveniste, and R. Black. 1995. Prevention of SIV infection in macaques by (R)-9-(2-phosphonylmethoxypropyl)adenine. Science 270:1197-1199. [DOI] [PubMed] [Google Scholar]
- 16.Tsai, C. C., K. E. Follis, T. W. Beck, A. Sabo, N. Bischofberger, and P. J. Dailey. 1997. Effects of (R)-9-(2-phosphonylmethoxypropyl)adenine monotherapy in chronic SIV infection in macaques. AIDS Res. Hum. Retrovir. 13:707-712. [DOI] [PubMed] [Google Scholar]
- 17.Van Rompay, K. K., J. M Cherrington, M. L. Marthas, C. J. Berardi, A. S. Mulato, A. Spinner, R. P. Tarara, D. R. Canfield, S. Telm, N. Bischofberger, and N. C. Pedersen. 1996. 9-[2-(Phosphonomethoxy)propyl]adenine therapy of established simian immunodeficiency virus infection in infant rhesus macaques. Antimicrob. Agents Chemother. 40:2586-2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Rompay, K. K., J. M Cherrington, M. L. Marthas, P. D. Lamy, P. J. Dailey, D. R. Canfield, R. P. Tarara, N. Bischofberger, and N. C. Pedersen. 1999. 9-[2-(Phosphonomethoxy)propyl]adenine (PMPA) therapy prolongs survival of infant macaques inoculated with simian immunodeficiency virus with reduced susceptibility to PMPA. Antimicrob. Agents Chemother. 43:802-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Rompay, K. K., C. J. Berardi, N. L. Aguirre, N. Bischofberger, P. S. Leitman, N. C. Pedersen, and M. L. Marthas. 1998. Two doses of PMPA protect newborn macaques against oral simian immunodeficiency virus infection. AIDS 12:F79-F83. [DOI] [PubMed] [Google Scholar]
- 20.Wainberg, M. A., M. D. Miller, Y. Quan, H. Salomon, A. S. Mulato, P. D. Lamy, N. A. Margot, K. E. Anton, and J. M. Cherrington. 1999. In vitro selection and characterization of HIV-1 with reduced susceptibility to PMPA. Antivir. Ther. 4:87-94. [DOI] [PubMed] [Google Scholar]