Abstract
Plasma antimalarial activity following oral artesunate or dihydroartemisinin (DHA) treatment was measured by a bioassay in 18 patients with uncomplicated falciparum malaria. The mean antimalarial activity in terms of the bioavailability of DHA relative to that of artesunate did not differ significantly from 1, suggesting that DHA can be formulated to be an acceptable oral alternative to artesunate.
Drug resistance to Plasmodium falciparum is a growing problem in Southeast Asia (14). The artemisinin derivatives are effective, safe, and widely used alternative treatments. Artesunate is the most commonly prescribed derivative (1, 7). Each artesunate treatment for adults costs at least $1 (U.S.). Nearly all the antimalarial activity of artesunate results from the activity of its main metabolite, dihydroartemisinin (DHA) (1, 2, 6). DHA has also been formulated as an oral antimalarial drug and could be easier and cheaper to produce than artesunate. Clinical trials on the treatment of acute, uncomplicated falciparum malaria have demonstrated that oral DHA is a safe, effective oral treatment that achieves cure rates similar to those achieved with oral artesunate (8, 9, 15).
A recent study with eight patients with uncomplicated falciparum malaria, in which high-performance liquid chromatography (HPLC) and UV detection (UVD) were used to determine the plasma DHA concentration, gave an estimated mean oral bioavailability of DHA relative to that of oral artesunate of 0.88 (4). Chemical methods for the assay of the artemisinin derivatives have a limit of accurate quantitation above the range of concentrations which provide a significant antimalarial effect. Bioassay gives an alternative and considerably more sensitive measure but does not distinguish between parent drugs and their active metabolites (12). We therefore compared the bioavailabilities of the two most widely used formulations of oral artesunate and oral DHA given at the same milligram doses to patients with uncomplicated malaria in a randomized crossover trial with bioassay measurements of antimalarial activity obtained with serial plasma samples (3, 10, 11, 12).
Nonpregnant, febrile (temperature, >37.5°C) adults (age, >14 years) hospitalized at the Mae Sot Hospital or the Mae La Camp of displaced Karen People, Tak Province, western Thailand, with uncomplicated acute P. falciparum malaria (defined as the presence of asexual stages of P. falciparum in peripheral blood and not fulfilling the World Health Organization [16] criteria for severe malaria) were included in the study, provided that they gave fully informed consent and they had not previously received significant antimalarial treatment. The study was approved by the ethical and scientific review subcommittee of the Thai Ministry of Public Health and by the Karen Refugee Committee. In the first 48 h the patients were given both artesunate (200 mg as 50-mg tablets; Guilin No. 1 Factory, Guangxi, People's Republic of China) and dihydroartemisinin (200 mg as 20-mg tablets; Cotecxin; Cotec New Technology Corp., Beijing, People's Republic of China). The patients were randomized in pairs to be given artesunate first or DHA first. The drug that they did not receive on the first day was administered 24 h later. The drug was administered approximately 2 h after a breakfast of rice soup. Heparinized blood samples were taken through an indwelling forearm vein catheter at 0, 5, 15, 30, 45, 60, 90, and 120 min and then 3, 4, 6, 8, 12, 18, and 24 h after the administration of each of the two doses. After 48 h the patient was given an additional 4 mg of oral artesunate per kg of body weight with 25 mg of mefloquine (Lariam; Roche) per kg (split as 15 mg/kg, followed by 10 mg/kg 24 h later) to complete the treatment.
Antimalarial activity in plasma was measured by an in vitro P. falciparum bioassay (with the chloroquine-resistant clone W2) in which antimalarial activity is expressed as DHA equivalents (12). The lower limit of quantitation of the bioassay was 11 nmol/liter; and the interassay coefficients of variation at 18, 44, and 176 nmol/liter were 13.4, 10.2, and 11.1%, respectively (n = 42). Dilutions were used for samples with concentrations >350 nmol/liter. The molecular weight of artesunate (384.4) is 35% greater than that of DHA (284.9), and the doses and concentrations in plasma of the study drugs were expressed in molar terms. The average percent potencies of artesunate and DHA tablets were determined by HPLC with electrochemical detection (ECD) and by bioassay. A sample from 20 crushed and dissolved tablets of each drug was analyzed to give the mean potency. Analyses were performed with SPSS software (version 8.0; SPSS Inc., Chicago, Ill.).
Twenty adults (two females) hospitalized with uncomplicated falciparum malaria were enrolled in the study. Two patients were excluded from the analysis. The patients presented after having been ill for a median of 3.5 days (range, 2.7 to 4.3 days) with fever, headache, anorexia, nausea, and vomiting. The median age was 24 years (range, 16 to 59 years), and the median body weight was 50.0 kg (range, 47.0 to 53.0 kg). The geometric mean admission level of parasitemia was 9,408/μl (95% confidence interval [CI] 3,142 to 28,170/μl; range, 150 to 237,635/μl). Although the milligram-per-kilogram doses were the same, in molar terms the median actual dose ingested was greater for DHA (14,656 nmol/kg; range 10,341 to 16,353 nmol/kg) than for artesunate (10,842 nmol/kg; range, 7,650 to 12,098 nmol/kg) (Table 1). Percent potencies by bioassay and HPLC-ECD were 87 and 88%, respectively, for artesunate tablets and 88 and 92%, respectively, for DHA tablets.
TABLE 1.
Median or mean doses and pharmacokinetic variables following artesunate and DHA administrationa
| Drug | Median (range) dose
|
Tlag (h)b | Tmax (h)b | Cmax (nmol/liter)b | k01(h−1)b | |
|---|---|---|---|---|---|---|
| mg/kg | nmol/kg | |||||
| DHA | 4.17 (2.94-4.65) | 14,656 (10,341-16,353) | 0.96 (0-2.91) | 2.63 (0.50-8.83) | 4,028 (1,904-16,018) | 0.656 (0.178-2.456) |
| Artesunate | 4.17 (2.94-4.65) | 10,842 (7,650-12,098) | 0.22 (0-1.46) | 1.55 (0.50-6.08) | 3,889 (1,011-12,691) | 8.128 (0.264-23.417) |
| P value | 0.023 | 0.006 | 0.25 | 0.2 | ||
| k10 (h−1)b | t1/2 (h)b | AUC0-∞ (nmol · h/liter)b | V/f (liters/kg)b | CL/f (liters/kg/h)b | AICb |
|---|---|---|---|---|---|
| 0.615 (0.176-1.118) | 1.09 (0.59-3.94) | 14,804 (7,116-38,225) | 1.36 (0.51-4.38) | 1.03 (0.35-1.87) | 166 (156-176) |
| 0.578 (0.192-1.342) | 1.17 (0.52-3.62) | 12,664 (3,275-21,489) | 1.38 (0.73-8.44) | 0.94 (0.49-2.34) | 165 (154-175) |
| 1.0 | 0.6 | 0.031 | 0.8 | 0.6 | 1.0 |
Each drug was administered to 18 patients. Abbreviations: Tlag, absorption lag time; k01, absorption rate constant; K10, elimination rate constant; t1/2 elimination half-life; V/f, total apparent volume of distribution per kilogram of body weight; f, fraction of the oral drug that is absorbed; AIC, Akaike information Criterion. The other abbreviations are defined in the text. Bioassay results are in DHA equivalents. To convert DHA equivalents from nanomoles per liter to nanograms per milliter, divide by 3.517.
Data are the means (95% CIs).
All patients made rapid and uncomplicated recoveries. There were no significant differences in clinical or laboratory features between those patients who received oral artesunate or DHA first. An open one-compartmental model gave a good fit for all except three of the data sets (WinNonlin, version 1.1; Scientific Consulting, Inc., Cary, N.C.); the three exceptions could be fitted only with a noncompartmental model. The area under the curve (AUC) from time zero to infinity (AUC0-∞) was calculated by using the linear trapezoid rule with log-linear extrapolation to infinity. Apparent oral clearance (CL/f) was calculated as dose/AUC0-∞
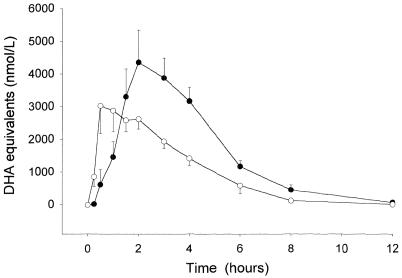
Artesunate was absorbed with a significantly shorter median lag time (P = 0.006) and earlier median peak antimalarial activity (P = 0.023) (Table 1; Fig. 1) than DHA. The median difference between the time to the maximum concentration in serum (Tmax) for patients treated with artesunate and DHA was 1.1 h (range, −4.5 to +8.2 h). The median maximum concentration of drug in serum (Cmax), corrected for molar dose/body weight, was 0.29 nmol/liter/nmol/kg (range, 0.14 to 1.21 nmol/liter/nmol/kg) after DHA treatment and 0.34 nmol/liter/nmol/kg (range, 0.09 to 1.22 nmol/liter/nmol/kg) after artesunate treatment (P = 0.9). Antimalarial activity fell to undetectable levels by 24 h after both treatments, confirming that a longer washout period after administration of the first dose was not required. The median antimalarial activity in terms of the AUC0-∞ was significantly greater after DHA administration than after artesunate administration (P = 0.03). This effect was due predominantly to the significantly higher DHA AUC0-∞ on the second day than on the first day (P = 0.04). The mean bioavailability of DHA relative to that of artesunate, with the dose expressed in molar units, was 1.20 (95% CI, 0.93 to 1.47) (12). This ratio does not differ significantly from 1 (P = 0.16 by the sign test). There were no significant relationships between any of the derived DHA and artesunate pharmacokinetic variables and no correlations with admission clinical and laboratory measurements (P > 0.05).
FIG. 1.
Mean (standard error) plasma antimalarial bioactivity in DHA equivalents following artesunate (○) and DHA (•) administration to patients with acute falciparum malaria.
There is little comparative pharmacokinetic information to guide the choice of the artemisinin derivative to be used in combination therapy (13). Oral artesunate is absorbed rapidly in patients with acute malaria, with absolute bioavailability averaging 0.61 (10). The absolute bioavailability of DHA cannot be determined directly, as there is no intravenous preparation suitable for humans. Artesunate is readily and almost completely hydrolyzed in vivo to DHA (1, 6), which has intrinsically greater antimalarial activity than artesunate. In terms of the current in vitro 50% inhibitory concentrations for P. falciparum in western Thailand, artesunate has ≈70% of the potency of DHA (5), although accurate comparisons are complicated by the hydrolysis of artesunate in solution, particularly at an acid pH.
The shorter lag time and time to maximum antimalarial activity (Tmax) after artesunate administration in comparison to that after DHA administration probably reflects the faster dissolution of the water-soluble artesunate tablet relative to the time of dissolution of the relatively insoluble DHA tablet. Excipients and drug particle size are also likely to be relevant, although this difference is unlikely to be important clinically in the oral treatment of uncomplicated malaria.
In a recent study of Vietnamese patients with uncomplicated falciparum malaria by HPLC-UVD assay, the mean oral DHA-artesunate bioavailability was 0.88 (95% CI, 0.49 to 1.27). This compares with the estimate of 1.20 (95% CI, 0.93 to 1.47) presented here, which was also not significantly different from 1. The two studies yielded similar Cmaxs, Tmaxs, AUCs, and elimination half-lives (4).
The mean antimalarial bioavailability, in molar units, of oral artemether relative to that of artesunate in Thai adults is 0.58 (95% CI, 0.40 to 0.76) (11), suggesting on pharmacokinetic grounds alone that artesunate rather than artemether would be the drug of choice for use in the combination treatment of uncomplicated malaria. However, the results from this subsequent study demonstrate that DHA and artesunate in these two widely used oral formulations have equivalent antimalarial activities in terms of their bioavailabilities and similar interindividual variabilities. As DHA is potentially cheaper and easier to manufacture than artesunate, these observations suggest that DHA could be a satisfactory alternative to artesunate for inclusion in combination treatments of uncomplicated falciparum malaria.
Acknowledgments
We are very grateful to the director and nursing and medical staff of Mae Sot Hospital, SMRU, Mae La, and to Kamolrat Silamut, Thanongsak Teewarakulpana, Kasia Stepniewska, and Kyaw Lay for help.
The bioassay was supported by the U.S. Army Medical Component, Armed Forces Research Institute of Medical Science, Bangkok, Thailand, and the U.S. Army Medical Research and Materiel Command, Fort Detrick, Frederick, Md. Michele van Vugt was supported by the Division of Infectious Diseases, Tropical Medicine and AIDS, Academic Medical Centre, University of Amsterdam, Amsterdam, The Netherlands. This study was part of the Wellcome Trust Mahidol University Oxford Tropical Medicine Research Programme funded by The Wellcome Trust of Great Britain
REFERENCES
- 1.Barradell, L. B., and A. Fitton. 1995. Artesunate: a review of its pharmacology and therapeutic efficacy in the treatment of malaria. Drugs 50:714-741. [DOI] [PubMed] [Google Scholar]
- 2.Batty, K. T., L. T. A. Thu, T. M. E. Davis, K. F. Illett, T. X. Mai, N. C. Hung, N. P. Tien, S. M. Powell, H. V. Thien, T. Q. Binh, and N. V. Kim. 1998. A pharmacokinetic and pharmacodynamic study of intravenous vs oral artesunate in uncomplicated falciparum malaria. Br. J. Clin. Pharmacol. 45:123-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bethell, D. B., P. Teja-Isavadharm, C. X. Phuong, P. T. Thuy, T. T. Mai, T. T. Thuy, N. T. Ha, P. T. Phuong, D. Kyle, N. P. J. Day, and N. J. White. 1997. Pharmacokinetics of oral artesunate in children with moderately severe Plasmodium falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 91:195-198. [DOI] [PubMed] [Google Scholar]
- 4.Binh, T. Q., K. F. Illett, K. T. Batty, T. M. E. Davis, N. C. Hung, S. M. Powell, L. T. A Thu, H. V. Thien, H. L. Phuong, and V. D. B. Phuong. 2001. Oral bioavailability of dihydroartemisinin in Vietnamese volunteers and in patients with falciparum malaria. Br. J. Clin. Pharmacol. 51:541-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brockman, A., R. N. Price, M. van Vugt, D. G. Heppner, D. Walsh, P. Sooklo, T. Wimonwaltrawatee, S. Looareeswuan, N. J. White, and F. Nosten. 2000. Plasmodium falciparum antimalarial drug susceptibility on the northwestern border of Thailand during five years of extensive use of artesunate-mefloquine. Trans. R. Soc. Trop. Med. Hyg. 94:537-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grace, J. M., D. J. Skanchy, and A. J. Aguilar. 1998. Metabolism of artelinic acid to dihydroqinghaosu by human liver cytochrome P450 3A. Xenobiotica 29:703-717. [DOI] [PubMed] [Google Scholar]
- 7.Hien, T. T., and N. J. White. 1993. Qinghaosu. Lancet 341:603-608. [DOI] [PubMed] [Google Scholar]
- 8.Looareesuwan, S., P. Wilairatana, S. Vanijanonta, P. Pitisuttithum, and C. Viravan. 1996. Treatment of acute, uncomplicated, falciparum malaria with oral dihydroartemisinin. Ann. Trop. Med. Parasitol. 90:21-28. [DOI] [PubMed] [Google Scholar]
- 9.Na-Bangchang, K., P. Tippanankosol, R. Ubalee, S. Chaovanakawee, S. Saenglertsilapachai, and J. Karbwang. 1999. Comparative clinical trial of four regimes of dihydroartemisinin-mefloquine in multidrug resistant falciparum malaria. Trop. Med. Int. Health 4:602-610. [DOI] [PubMed] [Google Scholar]
- 10.Newton, P. N., Y. Suputtamongkol, P. Teja-Isavadharm, S. Pukrittayakamee, I. Bates, V. Navaratnam, and N. J. White. 2000. Antimalarial bioavailability and disposition of artesunate in acute falciparum malaria. Antimicrob. Agents Chemother. 44:972-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suputtamongkol, Y., P. N. Newton, B. Angus, P. Teja-Isavadharm, D. Keeratithakul, M. Rasameedoraj, S. Pukrittayakamee, and N. J. White. A comparison of oral artesunate and artemether antimalarial bioactivities in acute falciparum malaria. Br. J. Clin. Pharmacol., in press. [DOI] [PMC free article] [PubMed]
- 12.Teja-Isavadharm, P., F. Nosten, D. E. Kyle, C. Luxemberger, F. ter Kuile, J. O. Peggins, T. G. Brewer, and N. J. White. 1996. Comparative bio-availability of oral, rectal, and intramuscular artemether in healthy subjects—use of simultaneous measurement by high performance liquid chromatography with electrochemical detection and bioassay. Br. J. Clin. Pharm. 42:599-604. [DOI] [PubMed] [Google Scholar]
- 13.White, N. J. 1996. Malaria, p. 1087-1164. In G. Cook (ed.), Manson's tropical diseases. The W. B. Saunders Co., London, United Kingdom.
- 14.White, N. J., F. Nosten, S. Looareesuwan, W. M. Watkins, K. Marsh, R. W. Snow, G. Kokwaro, J. Ouma, T. T. Hien, M. E. Molyneux, T. E. Taylor, C. I. Newbold, T. K. Reubush, M. Danis, B. M. Greenwood, R. M. Anderson, and P. Olliaro. 1999. Averting a malaria disaster. Lancet 353:1965-1967. [DOI] [PubMed] [Google Scholar]
- 15.Wilairatana, P., P. Chanthavanich, P. Singhasivanon, S. Treeprasertsuk, S. Krudsood, K. Chalermrut, C. Phisalaphong, K. Kraisintu, and S. Looareesuwan. 1998. A comparison of three different dihydroartemisinin formulations for the treatment of acute uncomplicated falciparum malaria in Thailand. Int. J. Parasitol. 28:1213-1218. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. 1990. Severe and complicated malaria. Trans. R. Soc. Trop. Med. Hyg. 84(Suppl. 2):1-65. [PubMed] [Google Scholar]