Abstract
Proteolytic processing of the abundant plasmatic human CC chemokine 1 (HCC-1) generates a truncated form, HCC-1[9-74], which is a potent agonist of CCR1, CCR3, and CCR5; promotes calcium influx and chemotaxis of T lymphoblasts, monocytes, and eosinophils; and inhibits infection by CCR5-tropic human immunodeficiency virus type 1 (HIV-1) isolates. In the present study we demonstrate that HCC-1[9-74] interacts with the second external loop of CCR5 and inhibits replication of CCR5-tropic HIV-1 strains in both primary T cells and monocyte-derived macrophages. Low concentrations of the chemokine, however, frequently enhanced the replication of CCR5-tropic HIV-1 isolates but not the replication of X4-tropic HIV-1 isolates. Only HCC-1[9-74] and HCC-1[10-74], but not other HCC-1 length variants, displayed potent anti-HIV-1 activities. Fluorescence-activated cell sorter analysis revealed that HCC-1[9-74] caused up to 75% down-regulation of CCR5 cell surface expression, whereas RANTES (regulated on activation, normal T-cell expressed and secreted) achieved a reduction of only about 40%. Studies performed with green fluorescent protein-tagged CCR5 confirmed that both HCC-1[9-74] and RANTES, but not full-length HCC-1, mediated specific internalization of the CCR5 HIV-1 entry cofactor. Our results demonstrate that the interaction with HCC-1[9-74] causes effective intracellular sequestration of CCR5, but they also indicate that the effect of HCC-1[9-74] on viral replication is subject to marked cell donor- and HIV-1 isolate-dependent variations.
Human immunodeficiency virus (HIV) type 1 (HIV-1), the main causative agent of AIDS, infects target cells by binding of its extracellular glycoprotein gp120 to the cellular CD4 receptor and subsequent interactions with chemokine receptors that allow fusion between the viral and cellular membranes (4, 11, 20, 28). CCR5 (R5) is the major entry cofactor for primary HIV-1 isolates and plays an important role in viral transmission. Variants capable to use another HIV-1 entry cofactor, CXCR4 (X4), emerge in about 50% of infected individuals during late stages of infection (4). However, use of R5 is sufficient for AIDS progression (9).
The ligands of the chemokine receptors are able to inhibit infection with the respective HIV-1 isolates. It has been shown that R5-mediated entry is blocked by its ligands RANTES (regulated on activation normal T-cell expressed and secreted), macrophage inflammatory protein 1α (MIP-1α), and MIP-1β (7). Most studies on antiviral drug development focused on RANTES, because it is a more effective inhibitor than MIP-1α and MIP-1β. A number of variants of RANTES with modified N termini and potent anti-HIV activities have been generated (39), and some entry inhibitors are being evaluated in clinical trials (27). Anti-HIV therapeutics aimed at blocking R5-mediated infection will probably be well tolerated, because individuals homozygous for the Δ32 R5 allele do not show any disease symptoms (32). However, some concerns do exist about the use of RANTES derivatives as agents against HIV-1. The inhibitory effects of RANTES and RANTES analogues on HIV-1 replication vary, depending on the cell lines, target cell donors, and the R5-tropic HIV-1 strains used (8, 25, 41). Furthermore, these CC chemokines can also stimulate viral infection via different mechanisms that are partly independent of the process of HIV-1 cell fusion (14, 36, 41). Therefore, the identification and characterization of additional R5 ligands that block HIV-1 infection remain important and might lead to the development of improved therapeutic agents.
Recently, it has been shown that mature hemofiltrate CC chemokine-1 (HCC-1) (37) represents an inactive precursor that is converted into the active chemokine by proteolytic processing (10). Full-length mature HCC-1 is a weak ligand for CCR1 and a weak activator of monocytes (44). It consists of 74 amino acids and shares about 46% sequence identity with MIP-1α and MIP-1β. HCC-1 is expressed on a wide variety of tissues and is present at concentrations of about 10 nM in human plasma. By use of an aequorin-based bioassay with an R5-expressing cell line, a truncated form of HCC-1 that lacks the first 8 amino acids was isolated from human hemofiltrate. Unlike HCC-1, the processed form, HCC-1[9-74], was a high-affinity agonist on CCR1 and R5 and, to a lesser extent, CCR3. Consistent with this, HCC-1[9-74] promoted calcium influx and the migration of T lymphocytes, monocytes, and eosinophils (10).
It has been shown that HCC-1[9-74] inhibits replication of the R5-tropic YU2 and JR-CSF isolates in peripheral blood mononuclear cells (PBMCs) and, unlike RANTES, does not enhance infection with X4-tropic HIV-1 strains in a reporter cell line (10). Little if anything is known, however, about the effects of HCC-1[9-74] on various R5-tropic HIV-1 isolates, on viral replication in monocyte-derived macrophages (MDMs), or on the specificity of R5 binding or about its antiviral mechanism. In the present study we demonstrate that HCC-1[9-74] can have differential effects on HIV-1 replication in PBMC and MDM cultures, depending on the chemokine concentration, the viral isolate, and the target cell donor. We also show that only HCC-1[9-74] and HCC-1[10-74], but not other HCC-1 length variants, efficiently block HIV-1 replication. Furthermore, our results demonstrate that HCC-1[9-74] interacts with the second extracellular loop (ECL-2) of R5 and induces R5 down-modulation from the cell surface.
MATERIALS AND METHODS
Synthesis of chemokines.
HCC-1[9-74] was prepared by 9-fluorenylmethoxy carbonyl solid-phase peptide synthesis, purified by reversed-phase high-pressure liquid chromatography, and characterized as described previously (10, 12).
HIV-1 clones and env genes.
Molecular HIV-1 clones and env genes from primary isolates were obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health: pYU2 (22) and pSG3.1 (13) from S. Ghosh, B. Hahn, and G. Shaw and pYK-JRCSF (6, 18) from I. S. Y. Chen and Y. Koyanagi. The P51-S env construct (15) was kindly provided by A. Werner (Langen, Germany).
Generation of HIV-1 NL4-3 V3 loop variants.
Splice-overlap extension PCR was used to replace the V3-coding sequence of the NL4-3 env with the corresponding region derived from primary HIV-1 isolates (Table 1). Briefly, the NL4-3 env sequences upstream of the V3 region were amplified with primers pAP-Stu5 (5′-TAGATAATACCAGCTATAGGT-3′) and pAP-Vb (5′-GTACAATTAATTTCTACAGATGTGTT-3′); and the V3 regions of the 005PF135, 011JR103, 92TH014-12, 93BR020-17, and P51-S env genes were amplified with primers pAP-Va (5′-TCTGTAGAAATTAATTGTACRAGRCCC-3′) and pAP-Nhe3 (5′-TAATTTGCTAGCTATCTGTTTTAAAGTGGCATTCCATTTTGCTCTACTAATGTTACAATGTGC-3′). The PCR products were gel purified, mixed, and subjected to a second PCR with primers pAP-Stu5 and pAP-Nhe3. The resulting fragments were cloned into the NL4-3 provirus by using the StuI and NheI restriction sites flanking the V3-coding region. All PCR-derived portions were sequenced to confirm that no additional mutations were present.
TABLE 1.
V3 loop sequence and biological properties of the HIV-1 variants analyzeda
| Isolate | V3 loop sequence | Charge | Replication
|
Coreceptors
|
||
|---|---|---|---|---|---|---|
| PM1 | PBMC | X4 | R5 | |||
| N143 | ------------r---------v-i-k-.-nm----- | +8 | +++ | +++ | +++ | − |
| 005PF135 | ----------g---..--------------------- | +5 | ++++ | +++ | − | +++ |
| 011JR103 | ------------p-..--------------------- | +5 | + | +++ | − | ++ |
| 92TH014-12 | -------------l..-----w----q---------- | +5 | +++ | +++ | − | +++ |
| 93BR020-17 | ----------r-sl..----v---a--------k--- | +7 | ++ | ++ | + | ++ |
| P51-S | ----g-k--r-ms-..------ia-rq------k--- | +8 | ++ | +++ | ++ | + |
| JR-CSF | ----S---------..--------------------- | +5 | ++ | ++ | − | +++ |
| YU2 | ------------n-..-----1--------------- | +5 | ++ | ++ | − | +++ |
| SG3-1 | -----kk---r-tt..----vy------v-------- | +8 | ++ | +++ | +++ | − |
| Consensus | CTRPNNNTRKSIHIQRGPGRAFYTTGEIIGDIRQAHC | +5 | ||||
All sequences are shown in comparison to the V3 consensus sequence. Dashes indicate amino acid identity, and dots indicate gaps introduced to optimize the alignment. The 005PF135, 011JR103, 92TH014-12, 93BR020-17, and P51-S env recombinants differ from parental X4-tropic isolate NL4-3. only in the V3 sequence. JR-CSF, YU2, and SG3-1 are previously described molecular clones (6, 13, 18, 22). The symbols represent highly efficient (+++), efficient (++), low (+), and no significant (−) replication or entry.
Cells, virus stocks, and infectivity assays.
PM1 cells (23), provided by M. Reitz through the NIH AIDS Research and Reference Reagent Program and were kept in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS). X4.15 and R5.3 cells were cultured in Dulbecco modified Eagle medium with 10% FCS and 100 μg of puromycin per ml. These cell lines coexpress CD4 and R5 or X4 and contain the β-galactosidase gene under the control of the HIV-1 promoter. They were generated from U373MGCD4 cells essentially as described previously (19), except that they were selected for high receptor expression levels. Human PBMCs were isolated by use of lymphocyte separation medium (Organon Teknika Corporation, Durham, N.C.) and were stimulated with phytohemagglutinin (4 μg/ml; Sigma) for 3 days in RPMI 1640 medium supplemented with 20% FCS and 100 U of interleukin 2 (IL-2) per ml. Virus stocks were generated as described previously (10), and the p24 antigen levels were quantitated with an HIV p24 enzyme-linked immunosorbent assay kit obtained through the AIDS Research and Reference Reagent Program. PM1 cells were infected with virus stocks containing 50 ng of p24 antigen, and PBMCs were infected with virus stocks containing with 20 ng of p24 antigen. The supernatant was collected at 3- or 4-day intervals from the infected cell cultures, and virus production was measured by a reverse transcriptase (RT) assay as described previously (10). To determine the HIV-1 coreceptor tropism, X4.15 and R5.3 cells were seeded on 48-well dishes and infected with virus containing 150 ng of p24 antigen. Infectivity was quantitated with a Galacto-Light Plus chemiluminescence reporter assay kit (Tropix, Bedford, Mass.), as recommended by the manufacturer. HIV-1 infection of P4-CCR5 cells was performed as described previously (10).
Chemokine inhibition of HIV-1 replication.
A total of 2.5 × 104 prestimulated PBMCs in assay medium (RPMI 1640 medium, 20% FCS, 50 U of IL-2 per ml) were seeded on 96-well dishes and incubated with the different concentrations of the chemokines for 2 h. Subsequently, the cells were infected with virus stocks containing 20 ng of p24 antigen in a total volume of 200 μl. The PBMCs were maintained in RPMI 1640 medium supplemented with 20% FCS and 50 U of IL-2 per ml and the same dose of chemokines. For MDM isolation, mononuclear cells were isolated from fresh blood as described above. A total of 2.5 × 105 cells were seeded in 96-well dishes and were cultured for 1 week in RPMI 1640 medium supplemented with 10% human serum (Gibco BRL), 50 U of granulocyte-macrophage colony-stimulating factor (Boehringer, Mannheim, Germany) per ml, and antibiotics to allow monocyte differentiation and adherence to the plates. Subsequently, nonadherent cells were removed by extensive washing with phosphate-buffered saline (PBS). The macrophages were treated with chemokines and infected as described above, except that virus stocks containing 1 ng of p24 antigen were used for infection. Supernatant was collected at regular intervals to monitor virus production by the RT assay. All primary cells used in this study were derived from donors containing two intact R5 alleles.
Binding assays.
Soluble HCC-1[9-74] was iodinated with iodogen (Pierce). Specific activities of 500 to 2,000 Ci/mmol were obtained by using 5 μg of protein with 500 μCi of Na125I for 20 min in 5-ml glass tubes precoated with 10 μg of iodogen by chloroform evaporation of a 100-μl volume of Iodogen under a slow stream of nitrogen gas. Radiolabeled proteins were purified from free Na125I by separation through a 0.3-ml Dowex column prepared in a 1-ml syringe and preequilibrated in a mixture containing 50 mM HEPES (pH 7.4), 5 mM MgCl2, 1 mM CaCl2, 1% bovine serum albumin, and 150 mM NaCl. Protein fractions were eluted in the void volume of the column, and fractions containing peaks of labeled protein were combined. 293T cells were transiently transfected with coreceptor expression plasmids by the calcium phosphate method, as described previously (33). Two days posttransfection the cells were washed twice with PBS and binding assays were performed by resuspending the cells in 75 μl of HEPES 11 binding buffer (50 mM HEPES [pH 7.4], 5 mM MgCl2, 1 mM CaCl2, 5% bovine serum albumin, 0.1% NaN3, 150 mM NaCl). Labeled peptide was added to cells in 25 μl of binding buffer for a total volume of 100 μl, and cells were incubated at room temperature for 1 h. For the competition studies the cells were preincubated with the doses of the monoclonal antibodies (MAbs) indicated below. Unbound radioactivity was removed by filtering the cells through 25-μm-pore-size Whatman GF/C filters presoaked in 0.2% polyethyleneimine (Sigma) and washing them two times with 4 ml of binding buffer. The counts on the filters were determined in a Wallac 1470 Wizard gamma counter.
FACS analysis.
GHOST (03) Hi-5 cells, provided by V. N. KewalRamani and D. R. Littman through the AIDS Research and Reference Reagent Program, were mixed with culture medium containing the indicated concentration of chemokines in a total volume of 100 μl. R5-negative parental HOS cells were included as a control. The reaction mixtures were incubated for 45 min at 37°C. Thereafter, the cells were transferred to 4°C and unbound chemokines were removed by washing with cold PBS. For R5 detection, the cells were mixed with R5-specific MAb 182 (R&D Systems) in a total volume of 100 μl of fluorescence-activated cell sorter (FACS) buffer. The reaction mixtures were incubated at 4°C for 1 h and washed, and bound antibody was detected by adding 3 μg of a phycoerythrin-coupled secondary antibody (Dianova). Antibody binding was allowed to proceed for 1 h at 4°C, and then the cells were washed and R5 expression was analyzed by FACS analysis. The relative efficiency of R5 down-modulation was determined as described previously (24).
Fluorescence microscopy.
HeLa cells were transiently transfected with constructs expressing R5 or X4 fused to the green fluorescent protein (GFP) (40) and were cultured on glass coverslips for 48 h. Subsequently, the cells were treated with cycloheximide for 2 h (50 mg/ml) to block new protein synthesis and to ensure that all receptor-GFP hybrid proteins have been transported to the cell surface. Thereafter, cells were treated with 100 μg of either RANTES, HCC-1, or HCC-1[9-74] per ml for 45 min in Dulbecco modified Eagle medium supplemented with 10% FCS at 37°C. All cells were then fixed in PBS containing 3% paraformaldehyde, and the coverslips were mounted in mowiol (Calbiochem). Images were captured with a cooled MicroMax charge-coupled device camera (Princeton Instruments). Image analysis and presentation were performed with IPLab Spectrum and Adobe Photoshop software.
RESULTS
HCC-1[9-74] and RANTES block R5-tropic HIV-1 replication with comparable efficiencies.
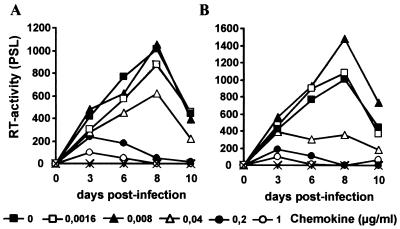
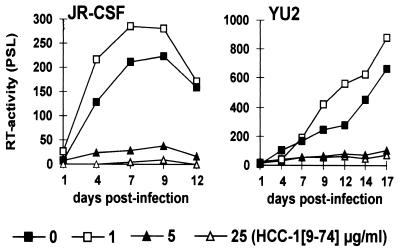
First, we compared the effects of HCC-1[9-74] and RANTES on the replication of the R5-tropic HIV-1 YU2 clone in PBMC cultures. As shown in Fig. 1, both chemokines effectively suppressed YU2 replication at 0.2 μg/ml. In some experiments RANTES was slightly more active than HCC-1[9-74]. Overall, however, results derived from multiple experiments with different batches of chemokines, independent virus stocks, and PBMCs from various donors demonstrated that HCC-1[9-74] and RANTES blocked viral replication with comparable efficiencies (data not shown).
FIG. 1.
Effects of HCC-1[9-74] and RANTES on YU2 replication. Prestimulated human PBMCs were infected with HIV-1 YU2 virus stocks containing 20 ng of p24 antigen in the presence of the indicated concentrations of HCC-1[9-74] (A) or RANTES (B). Supernatant was harvested at regular intervals and analyzed for RT activity, as described in Materials and Methods. The results shown were obtained with PBMCs from one donor in a single experiment. The study was repeated eight times with different batches of chemokine. Usually, HCC-1[9-74] and RANTES blocked YU2 replication with comparable efficiencies (data not shown). PSL, photon-stimulated light emission; ×, uninfected cells.
Differential sensitivities of different HIV-1 isolates to HCC-1[9-74].
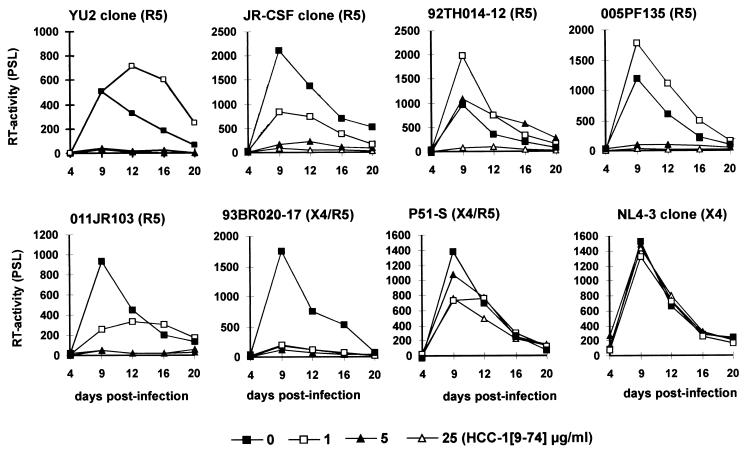
Changes in the V3 region of R5-tropic HIV-1 envelopes can modulate the sensitivity to chemokines without altering the coreceptor tropism (8, 25, 41). To investigate whether the inhibitory effect of HCC-1[9-74] is dependent on the V3 loop sequence, we generated a set of variants of X4-tropic clone NL4-3 (Table 1). Functional analysis revealed that three of these V3 recombinants (005PF135, 011JR103, and 92TH014-12) were R5 tropic and that two forms (93BR020-17 and P51-S) were dually tropic (Table 1). All forms replicated in PM1 cells and PBMCs. In addition, the sensitivities of the molecular YU2, JR-CSF, and SG3.1 HIV-1 clones to HCC-1[9-74] were examined.
As shown in Fig. 2, replication of the R5-tropic YU2 and JR-CSF clones was effectively blocked at concentrations of 5 and 25 μg/ml, respectively. In agreement with the results of a previous study (10), no inhibition of NL4-3 replication was observed. Similarly, HCC-1[9-74] did not inhibit replication of the SG3.1 clone, which uses X4 and GPR15 for entry into target cells (33), and an R5X4-tropic NL4-3 recombinant, which carries the V3 loop region of the P51-S isolate (Fig. 2 and data not shown). In contrast, replication of the X4R5-tropic 93BR020-17 recombinant was completely blocked at concentrations of 1 μg of HCC-1[9-74] per ml. These results are consistent with the finding that the P51-S recombinant preferentially uses X4 for entry into target cells, whereas the 93BR020-17 V3 variant predominantly uses R5 (Table 1). Also, the inhibitory effect of HCC-1[9-74] on different R5-tropic NL4-3 recombinants varied. At concentrations of 1 μg of HCC-1[9-74] per ml, replication of 011JR103 was partially inhibited, whereas replication of 92TH014-12 and 005PF135 was slightly enhanced (Fig. 2). Even at concentrations of 5 μg/ml, the 92TH014-12 recombinant showed efficient levels of replication, whereas the replication of all other R5-tropic HIV-1 isolates was blocked (Fig. 2). Full-length HCC-1 had no significant effect on viral replication (data not shown). These results demonstrate that the sensitivity of R5-tropic HIV-1 isolates to HCC-1[9-74] can be determined by the V3 loop sequence of envelope glycoprotein gp120.
FIG. 2.
Effects of HCC-1[9-74] on replication of different HIV-1 isolates in human PBMCs. Prestimulated PBMCs were exposed to the various HIV-1 isolates in the presence of the indicated concentrations of the chemokine. Virus stocks containing 20 ng of p24 antigen were used for infection. The results obtained with PBMCs from one donor in a single experiment are shown. Similar results were obtained in two independent experiments with PBMCs from different donors. Cells were cultured and virus production was measured by RT assay, as described in the legend to Fig. 1. PSL, photon-stimulated light emission.
Low concentrations of HCC-1[9-74] can enhance HIV-1 replication.
Several recent studies have shown that the effects of chemokines on viral replication are complex and that CC chemokines can have both inhibitory and stimulatory effects on HIV replication (2, 3, 7, 8, 25, 36, 39, 41). We observed that HCC-1[9-74] frequently enhances replication of R5-tropic HIV-1 isolates at relatively low concentrations (see the results, e.g., for 92TH014-12 and 005PF135 in Fig. 2).
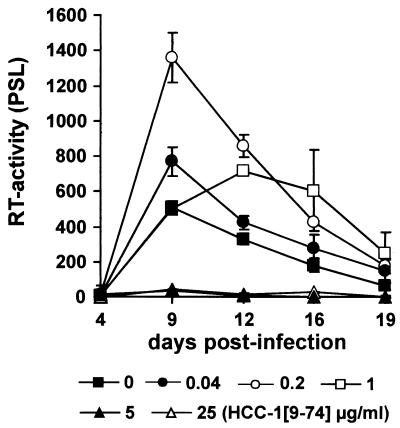
To further assess the effect of HCC-1[9-74] on viral replication, human PBMCs were infected in triplicate with the R5-tropic YU2 strain in the presence of various concentrations of the chemokine. As shown in Fig. 3, low concentrations of HCC-1[9-74] consistently increased the level of YU2 replication. At 0.04 μg/ml the peak RT activities in the PBMC culture supernatants were increased about 1.4-fold compared to the levels of replication observed in the absence of the chemokine, and at 0.2 μg/ml they were even increased 2.4-fold. The stimulatory effect at 0.2 μg/ml was statistically significant (P < 0.05). In comparison, the RT activities were essentially identical in the presence of the chemokine at 1 μg/ml at 9 days postinfection, when the peak was observed in the absence of HCC-1[9-74]. Thereafter, however, slightly increased activities were measured. In contrast, YU2 replication was completely blocked at concentrations of 5 and 25 μg of HCC-1[9-74] per ml.
FIG. 3.
Low concentrations of HCC-1[9-74] can increase viral replication. PBMCs from a single donor were infected in triplicate with normalized amounts (20 ng) of the R5-tropic YU2 isolate in the presence of the indicated amounts of HCC-1[9-74]. The data represent the average values and standard deviations. Cells were cultured and virus production was measured by RT assay, as described in the legend to Fig. 1. Similar results were obtained in independent experiments and with the R5-tropic JR-CSF isolate. PSL, photon-stimulated light emission.
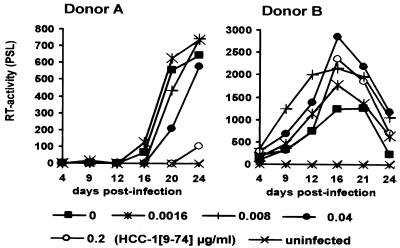
Next, we investigated if the stimulatory effects of low doses of HCC-1[9-74] on YU2 replication are dependent on the PBMC donor. As shown in Fig. 4, low concentrations of HCC-1[9-74] had little if any stimulatory effect on HIV-1 replication in PBMCs derived from donor A, and YU2 replication was efficiently blocked by the chemokine at 0.2 μg/ml. In contrast, viral replication in PBMCs derived from donor B, which were infected in parallel, was enhanced (Fig. 4). Donor-to-donor variations were also observed in independent experiments with PBMCs derived from 10 different blood donors and with the R5-tropic JR-CSF isolate (data not shown). Thus, the overall effect of HCC-1[9-74] on replication of R5-tropic HIV-1 in PBMC cultures is dependent on the viral isolate used, the PBMC donor, and the chemokine concentration. Relatively high concentrations of HCC-1[9-74] block HIV-1 replication, whereas low concentrations might stimulate viral replication. Notably, the effects of low doses of chemokines on HIV-1 replication in PBMCs were specific for R5-tropic isolates. At concentrations ≤0.2 μg/ml we did not observe a significant effect of HCC-1[9-74] or RANTES on replication of X4-tropic HIV-1 strains (data not shown). At concentrations ≥1.0 μg/ml, RANTES but not HCC-1[9-74] enhanced infection by X4-tropic HIV-1 isolates (10) (data not shown).
FIG. 4.
The effects of low doses of HCC-1[9-74] on YU2 replication depend on the PBMC donor. PBMCs from donors A and B were infected, and virus production was measured by the RT assay, as described in the legend to Fig. 1. Average values obtained from triplicate infections are shown. Donor-dependent variations were confirmed in five independent experiments with PBMCs from 10 additional donors and different virus stocks. PSL, photon-stimulated light emission.
HCC-1[9-74] inhibits replication of R5-tropic HIV-1 isolates in macrophages.
The effects of RANTES on HIV-1 replication in macrophages are controversial, and both inhibitory and stimulatory effects have been described (2, 17, 37, 46). To investigate the effect of HCC-1[9-74] on viral replication in primary macrophages, we infected MDMs with isolates R5-tropic JR-CSF and YU2. HCC-1[9-74] had modest stimulatory effects on HIV-1 replication at concentrations of 0.2 and 1.0 μg/ml (Fig. 5 and data not shown). Higher concentrations of the chemokine, however, blocked replication of both HIV-1 isolates in MDM cultures. Thus, the effects of HCC-1[9-74] on HIV-1 replication in MDMs were similar to those observed in PBMCs: efficient inhibition at relatively high concentrations but stimulatory effects at low doses.
FIG. 5.
HCC-1[9-74] inhibits HIV-1 replication in macrophages. Macrophages were isolated from fresh blood by using lymphocyte separation medium as described in the Materials and Methods section. Cells were infected with virus stocks containing 1 ng of p24 antigen, and RT activities were measured as described in the legend to Fig. 1. Average values obtained from triplicate infections are shown. Comparable results were obtained in two independent experiments with MDMs derived from different donors. PSL, photon-stimulated light emission.
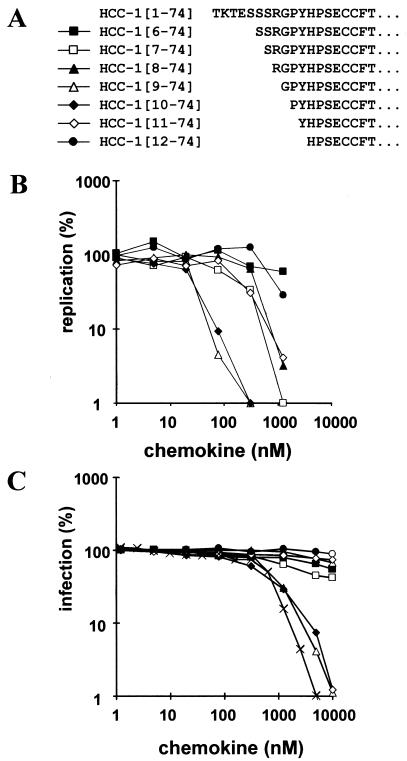
Only HCC-1[9-74] and HCC-1[10-74] are potent HIV-1 inhibitors.
In addition to HCC-1[9-74], a number of additional posttranslationally processed forms of HCC-1 have been detected in human plasma (34). To further define the role of N-terminal processing for the biological activity of this chemokine, a number of HCC-1 length variants starting at amino acid positions 6 through 12 were synthesized for functional analysis (Fig. 6A). Both HCC-1[9-74] and HCC-1[10-74] showed comparable antiviral activities and blocked HIV-1 YU2 replication in PBMCs with an efficiency >90% at a concentration of 78 nM (≅0.6 μg/ml; Fig. 6B). Similarly, these two variants also blocked YU2 infection of P4-CCR5 cells (Fig. 6C). RANTES was about twofold more active than HCC-1[9-74] and HCC-1[10-74] in this experiment. The remaining HCC-1 forms were much less effective in inhibiting HIV-1 replication or infection. The result that only HCC-1 forms starting at residues 9 and 10 are potent R5 agonists was confirmed in receptor binding studies and additional infection experiments with PBMCs from three different donors (data not shown). Our data imply that precise proteolytic processing is crucial for the full biological activity of HCC-1.
FIG. 6.
Effects of HCC-1 length variants on HIV-1 YU2 replication in PBMCs. (A) N-terminal sequences of the HCC-1 variants analyzed. (B) Human PBMCs were infected in triplicate with YU2 in the presence of the indicated chemokine concentrations (an HCC-1[9-74] concentration of 1 μg/ml corresponds to 128 nM). The data represent the average peak RT activity measured in cell culture supernatants 22 days after infection. The RT activity detected in the absence of chemokine was considered 100% replication efficiency. (C) P4-CCR5 cells were infected with the YU2 strain in the presence of the various chemokines, and infectivity was assayed as described previously (10). Average values obtained from triplicate infections are shown. ×, RANTES.
HCC-1[9-74] binds to ECL-2 of R5.
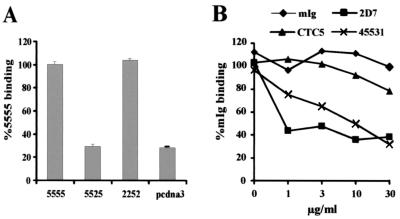
We used chimeras between the R5 and CCR2b molecules (21, 35) to investigate which domains in R5 are involved in HCC-1[9-74] binding. As shown in Fig. 7A, substitution of the ECL-2 domain of R5 with the corresponding region of CCR2b disrupted the interaction with HCC-1[9-74]. Conversely, replacement of the ECL-2 region of CCR2b with the ECL-2 domain of R5 resulted in efficient binding. To further analyze which domains in R5 are important for chemokine interaction, we investigated whether antibodies that bind to different epitopes in R5 disrupt binding of HCC-1[9-74]. Two antibodies, 2D7 and 45531, which recognize the ECL-2 of R5 (45) blocked HCC-1[9-74] binding (Fig. 7B). MAb 2D7 was more efficient than MAb 45531. This difference might be explained by the higher binding affinity of MAb 2D7 for R5 or by the fact that both MAbs recognize different residues in the ECL-2 region (20). In contrast, CTC5, which recognizes the NH2-terminal region of R5 (45), did not interfere with HCC-1[9-74] binding. The results obtained with both assay systems suggest that ECL-2 is the major determinant for HCC-1[9-74] binding by R5.
FIG. 7.
HCC-1[9-74] binds to ECL-2 of R5. (A) 293T cells were transfected with expression plasmids encoding R5 and CCR2b chimeras. Chimeras are designated by their extracellular domains as described previously (20): 5555, wild-type R5; 5525, a chimera containing the ECL-2 region of CCR2b; 2252, a CCR2b chimera containing the ECL-2 domain of R5. At 48 h posttransfection, the cells were harvested and processed for the binding assay, as described in Materials and Methods. The results of the experiment shown are representative of two performed in triplicate. The data are represented as means ± standard errors of the means. (B) Binding of HCC-1[9-74] to 293 cells stably transfected with R5 preincubated with MAbs that interact with the N terminus (MAb CTC5) or the ECL-2 region (MAbs 2DZ and 45531) of R5 (20, 45). The results of the experiment shown are representative of two performed. mIg, membrane immunoglobulin.
HCC-1[9-74] down-modulates R5 cell surface expression.
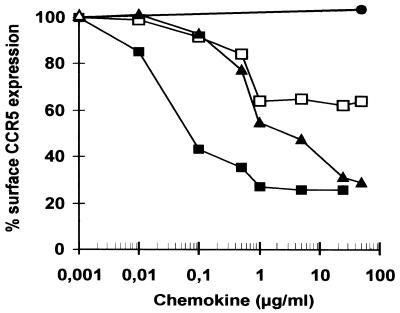
Chemokines might block HIV-1 infection by inhibition of the interaction between the viral gp120 and the coreceptor or by down-regulation of coreceptors from the cell surface (1, 3, 38, 39). To assess their effects on R5 cell surface expression, we treated GHOST (03) Hi-5 cells for 45 min with various concentrations of aminooxypentane (AOP)-RANTES, RANTES, HCC-1, and HCC-1[9-74]. R5 expression was measured by FACS analysis with R5-specific MAb 182. With the exception of full-length HCC-1, all chemokines reduced the level of R5 surface expression (Fig. 8). In agreement with previously published data (24), AOP-RANTES was very potent in R5 down-regulation. At 0.1 μg/ml it caused 50% down-regulation, and at concentrations ≥1 μg/ml, <30% of R5 receptors were detectable (Fig. 8). Although the natural ligands RANTES and HCC-1[9-74] also caused the disappearance of R5 from the cell surface, the effects were much weaker. At concentrations ≤1 μg/ml, both HCC-1[9-74] and RANTES showed comparable efficiencies in reducing R5 surface expression. However, HCC-1[9-74] was more effective than RANTES in reducing R5 surface expression at concentrations >1 μg/ml. Even at high concentrations RANTES achieved only about a 40% reduction. In contrast, the effect of HCC-1[9-74] on R5 cell surface expression did not saturate, and at 25 μg/ml, HCC-1[9-74] resulted in about 70% down-regulation, similarly to AOP-RANTES (Fig. 8).
FIG. 8.
Down-regulation of R5 from the surfaces of stable transfected GHOST (03) Hi-5 cells. HOS cells expressing CD4 and high levels of R5 were incubated with the indicated concentrations of the different chemokines. Cell surface R5 expression was detected with R5-specific MAb 182 and analyzed by FACS analysis. Values give the percentages of mean fluorescence intensity.
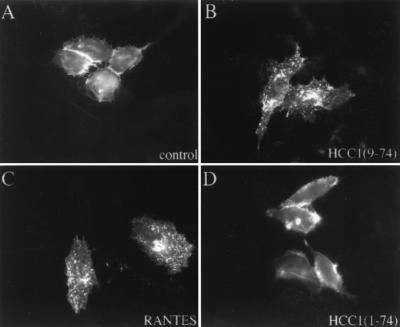
To exclude the possibility that treatment with HCC-1[9-74] interferes with the binding of MAb 182 to R5, we directly monitored the effect of HCC-1[9-74] on the cellular localization of R5. HeLa cells expressing an R5-GFP fusion protein were treated with the chemokines for 45 min at 37°C, fixed, and analyzed by fluorescence microscopy. In untreated cells, R5-GFP was localized predominantly at the cell surface (Fig. 9A). Incubation with both HCC-1[9-74] or RANTES caused receptor internalization, resulting in a prominent intracellular vesicular staining (Fig. 9B and C). In contrast, full-length HCC-1 had little effect on R5-GFP cell surface expression (Fig. 9D). None of the three chemokines caused internalization of a X4-GFP hybrid (data not shown). Thus, in agreement with the FACS analysis, HCC-1[9-74] caused efficient and specific internalization of R5.
FIG. 9.
Effects of chemokines on the subcellular localization of R5-GFP. HeLa cells transiently expressing R5-GFP were treated with medium alone (A) or with medium containing 100 μg of either HCC-1[9-74] (B), RANTES (C), or HCC-1 (D) per ml for 45 min at 37°C. After fixation the cells were examined by fluorescence microscopy.
DISCUSSION
Our results demonstrate that HCC-1[9-74] interacts with ECL-2 of R5, induces R5 internalization, and is an effective inhibitor of R5-tropic HIV-1 variants in human MDMs and PBMCs. Overall, the potency of HCC-1[9-74] for the inhibition of R5-tropic HIV-1 isolates was similar to that of RANTES. This observation is consistent with recent data, which show that both chemokines act with comparable efficiencies as high-affinity agonists of R5 (10). HCC-1[9-74], but not RANTES, completely desensitizes cells to further stimulation (10). This is notable because it has been suggested that the rank order among CC chemokines for inhibition of HIV-1 replication might be correlated with the sustained R5 desensitization after ligand binding (16).
HCC-1[9-74] inhibited replication of the NL4-3 011JR103 V3 recombinant in PBMC cultures about fivefold more effectively than the 92TH014-12 V3 mutant. Both variants showed comparable replicative capacities, and they differed by only 4 amino acid changes in the V3 loop region, but these changes did not alter the overall charge of the gp120 glycoproteins. These results are consistent with those from studies that showed that the differential sensitivities of R5-tropic HIV-1 variants against RANTES seem to depend on the envelope V3 loop sequence but not on the V3 charge or the replicative capacity of the viral isolate (8, 16, 25, 41, 42, 43). The exact residues critical for the variable sensitivities against chemokine inhibition remain to be identified. Additional comparative studies with RANTES- and HCC-1-based antagonists are required to investigate whether HIV variants that show reduced sensitivities to RANTES analogs are also less effectively blocked by HCC-1 analogs. Such experiments seem important to clarify whether combinations of these chemokine derivatives might achieve improved therapeutic effects and reduce the risk for the selection of R5-tropic HIV-1 variants that are resistant to R5 antagonists.
One concern about the use of RANTES-based antagonists for the treatment of HIV-1-infected individuals is that RANTES and AOP-RANTES can enhance HIV-1 infection (14, 42). This effect is, at least in part, independent of the mechanism of cell fusion (14). Thus, therapeutic approaches based on RANTES derivatives not only might favor the emergence of X4-tropic strains (29) but also might even stimulate their replication. We also observed that HCC-1[9-74] at low doses had an enhancing effect on viral replication in both PBMCs and MDMs. However, the stimulatory effects on viral replication in MDM cultures were weak compared to those observed in the presence of RANTES (data not shown). Furthermore, the HCC-1[9-74]-mediated enhancement of replication was specific for R5-tropic HIV-1 isolates. These results are in agreement with previous results showing that the enhancing effects of RANTES are not mimicked by other chemokines like MIP-1α and MIP-1β (14). Our results indicate that somewhat different mechanisms underlie the stimulatory effects of HCC-1[9-74] and RANTES on HIV-1 replication and suggest that HCC-1-based HIV-1 inhibitors might have an advantage over RANTES-based inhibitors because they might not stimulate infection with X4-tropic HIV-1 strains.
It has been proposed that both coreceptor internalization and blocking of the HIV env-coreceptor interaction contributes to the inhibitory effects of chemokines on HIV infection (1, 3, 26, 38, 39). We found that both HCC-1[9-74] and RANTES induce comparable levels of R5 down-modulation from the cell surface at low chemokine concentrations. At higher concentrations (>1 μg/ml), however, HCC-1[9-74] was more effective. The dose required for >50% R5 down-regulation on GHOST (03) Hi-5 cells was about 1 μg/ml (Fig. 8). The effect of the same concentration of HCC-1[9-74] varied, depending on the PBMC donor and the viral isolate. Nonetheless, the finding that comparable concentrations of the chemokine are required to block replication of R5-tropic isolates and to cause R5 down-modulation suggests that coreceptor internalization might contribute to inhibition by HCC-1[9-74]. Previous studies imply that ECL-2 of R5 is of major importance for the binding of RANTES, MIP-1α, and MIP-1β (20, 35, 45). We found that this region is also important for the interaction with HCC-1[9-74], suggesting that this single domain is of general relevance for chemokine binding. In contrast, several studies suggest that multiple domains in R5 are involved in gp120 binding. Some R5-tropic isolates seem to interact predominantly with the NH2 terminus and ECL-1 (45). Other strains, however, seem to interact with the ECL-2 region of R5 (5). It remains to be clarified whether interactions with different extracellular domains of R5 or dependencies on different coreceptor expression levels for cell entry contribute to the different susceptibilities of various R5-tropic HIV-1 isolates to HCC-1[9-74] inhibition.
Our results show that, depending on the chemokine concentration and the viral isolate, HCC-1[9-74] might exert different effects on HIV-1 replication in primary cells. Furthermore, similar to previous reports on RANTES (42), we observed substantial donor-to-donor variations in the effects of HCC-1[9-74] on replication of R5-tropic HIV-1 mutants in PBMCs or MDMs. Different R5 expression levels or variations in the extents of endogenous chemokines could explain these results (31). Proteases activated at sites of inflammation likely result in the generation of the active truncated HCC-1[9-74] chemokine from its inactive HCC-1 precursor (10). It is difficult to predict whether HCC-1[9-74] is present at concentrations that have inhibitory or activating effects on HIV-1 replication in infected individuals. HCC-1 is present at concentrations of up to 10 nM in the plasma of healthy humans and up to 80 nM in the plasma of patients with renal failure (37). The chemokine concentrations might be much higher, however, at sites of inflammation and in lymphatic tissues, the major site of HIV-1 replication in vivo (30). Experiments with ex vivo lymphatic tissues (26) might allow clarification of the net effects of HCC-1[9-74] and other chemokines in human tissues. Furthermore, well-controlled patient studies are required to elucidate a possible correlation of high levels of HCC-1[9-74] with delayed rates of disease progression. Such studies might clarify whether approaches aimed at induction of the conversion of the active chemokine from the relatively large pool of inactive HCC-1 precursor could have beneficial effects on the course of HIV-1 infection.
As a first step in the evaluation of the potential of HCC-1-based receptor antagonists as antiviral therapy, we have investigated some forms of HCC-1[9-74] modified at their amino termini. In analogy to modified forms of RANTES (29, 39), these HCC-1 derivatives displayed increased antiviral activities. Thus, HCC-1-based receptor antagonists might present suitable candidates for anti-HIV therapy and complement approaches based on other chemokine receptor antagonists.
Acknowledgments
We thank Mandy Krumbiegel and Nathalie Finze for expert technical assistance, Bernhard Fleckenstein and Thomas Mertens for constant support and encouragement, Ingrid Bennett for critical reading of the manuscript, and Marc Parmentier and Michel Detheux for helpful comments and communication of unpublished data. A number of reagents were obtained through the AIDS Research and Reference Program.
This work was supported by grants from the Deutsche Forschungsgemeinschaft (grant SFB 466), the Johannes and Frieda Marohn Foundation, the Wilhelm-Sander Foundation, and the German government (grant BMBF FKZ 0311815).
REFERENCES
- 1.Amara, A., S. Legall, O. Schwartz, J. Salamero, M. Montes, P. Loetscher, M. Baggiolini, J. L. Virelizier, and F. Arenza-Naseisdedos. 1997. HIV coreceptor down-regulation as antiviral principle: SDF-1α-dependent internalization of the chemokine receptor CXCR4 contributes to inhibition of HIV replication. J. Exp. Med. 186:139-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amzazi, S., L. Ylisastigui, Y. Bakri, L. Rabehi, L. Gattegno, M. Parmentier, J. C. Gluckman, and A. Benjouad. 1998. The inhibitory effect of RANTES on the infection of primary macrophages by R5 human immunodeficiency virus type-1 depends on the macrophage activation state. Virology 252:96-105. [DOI] [PubMed] [Google Scholar]
- 3.Arenzana-Seisdedos, F., J. L. Virelizier, D. Rousset, I. Clark-Lewis, P. Loetscher, B. Moser, and M. Baggiolini. 1996. HIV blocked by chemokine antagonist. Nature 383:400.. [DOI] [PubMed] [Google Scholar]
- 4.Berger, E. A., P. M. Murphy, and J. M. Farber. 1999. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 17:657-700. [DOI] [PubMed] [Google Scholar]
- 5.Bieniasz, P. D., R. A. Fridell, I. Aramori, S. S. Ferguson, M. G. Caron, and B. R. Cullen. 1997. HIV-1-induced cell fusion is mediated by multiple regions within both the viral envelope and the CCR-5 co-receptor. EMBO J. 16:2599-2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cann, A. J., J. A. Zack, A. S. Go, S. J. Arrigo, P. L. Green, Y. Koyanagi, S. Pang, and I. S. Chen. 1990. Human immunodeficiency virus type 1 T-cell tropism is determined by events prior to provirus formation. J. Virol. 64:4735-4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cocchi, F., A. L. DeVico, A. Garzino-Demo, S. K. Arya, R. C. Gallo, and P. Lusso. 1995. Identification of RANTES, MIP-1α, and MIP-1β as the major HIV-suppressive factors produced by CD8+ T cells. Science 270:1811-1815. [DOI] [PubMed] [Google Scholar]
- 8.Cocchi, F., A. L. DeVico, A. Garzino-Demo, A. Cara, R. C. Gallo, and P. Lusso. 1996. The V3 domain of the HIV-1 gp120 envelope glycoprotein is critical for chemokine-mediated blockage of infection. Nat. Med. 2:1244-1247. [DOI] [PubMed] [Google Scholar]
- 9.de Roda Husman, A. M., R. P. van Rij, H. Blaak, S. Broersen, and H. Schuitemaker. 1999. Adaptation to promiscuous usage of chemokine receptors is not a prerequisite for human immunodeficiency virus type 1 disease progression. J. Infect. Dis. 180:1106-1115. [DOI] [PubMed] [Google Scholar]
- 10.Detheux, M., L. Standker, V. Vakili, J. Münch, U. Forssmann, K. Adermann, S. Pöhlmann, G. Vassart, F. Kirchhoff, M. Parmentier, and W. G. Forssmann. 2000. Natural proteolytic processing of hemofiltrate CC chemokine 1 generates a potent CC chemokine receptor (CCR)1 and CCR5 agonist with anti-HIV properties. J. Exp. Med. 192:1501-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doms, R. W., and S. C. Peiper. 1997. Unwelcome guests with master keys: how HIV uses chemokine receptors for cellular entry. Virology 235:179-190. [DOI] [PubMed] [Google Scholar]
- 12.Escher, S. E., H. Sticht, W. G. Forssmann, P. Rösch, and K. Adermann. 1999. Synthesis and characterization of the human CC chemokine HCC-2. J. Peptide Res. 54:505-513. [DOI] [PubMed] [Google Scholar]
- 13.Ghosh, S. K., P. N. Fultz, E. Keddie, M. S. Saag, P. M. Sharp, B. H. Hahn, and G. M. Shaw. 1993. A molecular clone of HIV-1 tropic and cytopathic for human and chimpanzee lymphocytes. Virology 194:858-864. [DOI] [PubMed] [Google Scholar]
- 14.Gordon, C. J., M. A. Muesing, A. E. Proudfoot, C. A. Power, J. P. Moore, and A. Trkola. 1999. Enhancement of human immunodeficiency virus type 1 infection by the CC-chemokine RANTES is independent of the mechanism of virus-cell fusion. J. Virol. 73:684-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holtkamp, N., A. Otteken, S. Findhammer, V. Miller, R. Kurth, and A. Werner. 2000. Unexpected coreceptor usage of primary human immunodeficiency virus type 1 isolates from viremic patients under highly active antiretroviral therapy. J. Infect. Dis. 181:513-521. [DOI] [PubMed] [Google Scholar]
- 16.Jansson, M., E. Backstrom, A. Bjorndal, V. Holmberg, P. Rossi, E. M. Fenyo, M. Popovic, J. Albert, and H. Wigzell. 1999. Coreceptor usage and RANTES sensitivity of non-syncytium-inducing HIV-1 isolates obtained from patients with AIDS. J. Hum. Virol. 2:325-338. [PubMed] [Google Scholar]
- 17.Kelly, M. D., H. M. Naif, S. L. Adams, A. L. Cunningham, and A. R. Lloyd. 1998. Dichotomous effects of β-chemokines on HIV replication in monocytes and monocyte-derived macrophages. J. Immunol. 160:3091-3095. [PubMed] [Google Scholar]
- 18.Koyanagi, Y., S. Miles, R. T. Mitsuyasu, J. E. Merrill, H. V. Vinters, and I. S. Chen. 1987. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science 236:819-822. [DOI] [PubMed] [Google Scholar]
- 19.Labrosse, B., A. Brelot, N. Heveker, N. Sol, D. Schols, E. De Clercq, and M. Alizon. 1998. Determinants for sensitivity of human immunodeficiency virus coreceptor CXCR4 to the bicyclam AMD3100. J. Virol. 72:6381-6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee, B., J. Ratajczak, R. W. Doms, A. M. Gewirtz, and M. Z. Ratajczak. 1999. Coreceptor/chemokine receptor expression on human hematopoietic cells: biological implications for human immunodeficiency virus-type 1 infection. Blood 93:1145-1156. [PubMed] [Google Scholar]
- 21.Lee, B., M. Sharron, C. Blanpain, B. J. Doranz, J. Vakili, P. Setoh, E. Berg, G. Liu, H. R. Guy, S. R. Durell, M. Parmentier, C. N. Chang, K. Price, M. Tsang, and R. W. Doms. 1999. Epitope mapping of CCR5 reveals multiple conformational states and distinct but overlapping structures involved in chemokine and coreceptor function. J. Biol. Chem. 274:9617-9626. [DOI] [PubMed] [Google Scholar]
- 22.Li, Y., J. C. Kappes, J. A. Conway, R. W. Price, G. M. Shaw, and B. H. Hahn. 1991. Molecular characterization of human immunodeficiency virus type 1 cloned directly from uncultured human brain tissue: identification of replication-competent and -defective viral genomes. J. Virol. 65:3973-3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lusso, P., F. Cocchi, C. Balotta, P. D. Markham, A. Louie, P. Farci, R. Pal, R. C. Gallo, and M. S. Reitz, Jr. 1995. Growth of macrophage-tropic and primary human immunodeficiency virus type 1 (HIV-1) isolates in a unique CD4+ T-cell clone (PM1): failure to downregulate CD4 and to interfere with cell-line-tropic HIV-1. J. Virol. 69:3712-3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mack, M., B. Luckow, P. J. Nelson, J. Cihak, G. Simmons, P. R. Clapham, N. Signoret, M. Marsh, M. Stangassinger, F. Borlat, T. N. Wells, D. Schlondorff, and A. E. Proudfoot. 1998. Aminooxypentane-RANTES induces CCR5 internalization but inhibits recycling: a novel inhibitory mechanism of HIV infectivity. J. Exp. Med. 187:1215-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maeda, Y., M. Foda, S. Matsushita, and S. Harada. 2000. Involvement of both the V2 and V3 regions of the CCR5-tropic human immunodeficiency virus type 1 envelope in reduced sensitivity to macrophage inflammatory protein 1α. J. Virol. 74:1787-1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Margolis, L. B., S. Glushakova, J. C. Grivel, and P. M. Murphy. 1998. Blockade of CC chemokine receptor 5 (CCR5)-tropic human immunodeficiency virus-1 replication in human lymphoid tissue by CC chemokines. J. Clin. Investig. 101:1876-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michael, N. L., and J. P. Moore. 1999. HIV-1 entry inhibitors: evading the issue. Nat. Med. 5:740-742. [DOI] [PubMed] [Google Scholar]
- 28.Moore, J. P., A. Trkola, and T. Dragic. 1997. Co-receptors for HIV-1 entry. Curr. Opin. Immunol. 9:551-562. [DOI] [PubMed] [Google Scholar]
- 29.Mosier, D. E., G. R. Picchio, R. J. Gulizia, R. Sabbe, P. Poignard, L. Picard, R. E. Offord, D. A. Thompson, and J. Wilken. 1999. Highly potent RANTES analogues either prevent CCR5-using human immunodeficiency virus type 1 infection in vivo or rapidly select for CXCR4-using variants. J. Virol. 73:3544-3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pantaleo, G., C. Graziosi, J. F. Demarest, O. J. Cohen, M. Vaccarezza, K. Gantt, C. Muro-Cacho, and A. S. Fauci. 1994. Role of lymphoid organs in the pathogenesis of human immunodeficiency virus (HIV) infection. Immunol. Rev. 140:105-130. [DOI] [PubMed] [Google Scholar]
- 31.Paxton, W. A., R. Liu, S. Kang, L. Wu, T. R. Gingeras, N. R. Landau, C. R. Mackay, and R. A. Koup. 1998. Reduced HIV-1 infectability of CD4+ lymphocytes from exposed-uninfected individuals: association with low expression of CCR5 and high production of beta-chemokines. Virology 244:66-73. [DOI] [PubMed] [Google Scholar]
- 32.Paxton, W. A., S. R. Martin, D. Tse, T. R. O'Brien, J. Skurnick, N. L. VanDevanter, N. Padian, J. F. Braun, D. P. Kotler, S. M. Wolinsky, and R. A. Koup. 1996. Relative resistance to HIV-1 infection of CD4 lymphocytes from persons who remain uninfected despite multiple high-risk sexual exposure. Nat. Med. 2:412-417. [DOI] [PubMed] [Google Scholar]
- 33.Pöhlmann, S., M. Krumbiegel, and F. Kirchhoff. 1999. Coreceptor usage of BOB/GPR-15 and Bonzo/STRL-33 by primary HIV-1 isolates. J. Gen. Virol. 80:1241-1251. [DOI] [PubMed] [Google Scholar]
- 34.Richter, R., P. Schulz-Knappe, H. John, and W. G. Forssmann. 2000. Posttranslationally processed forms of the human chemokine HCC-1. Biochemistry 39:10799-10805. [DOI] [PubMed] [Google Scholar]
- 35.Rucker, J., M. Samson, B. J. Doranz, F. Libert, J. F. Berson, Y. Yi, R. J. Smyth, R. G. Collman, C. C. Broder, G. Vassart, et al. 1996. Regions in beta-chemokine receptors CCR5 and CCR2b that determine HIV-1 cofactor specificity. Cell 87:437-446. [DOI] [PubMed] [Google Scholar]
- 36.Schmidtmayerova, H., B. Sherry, and M. Bukrinsky. 1996. Chemokines and HIV replication. Nature 382:767.. [DOI] [PubMed] [Google Scholar]
- 37.Schulz-Knappe, P., H. J. Magert, B. Dewald, M. Meyer, Y. Cetin, M. Kubbies, J. Tomeczkowski, K. Kirchhoff, M. Raida, K. Adermann, et al. 1996. HCC-1, a novel chemokine from human plasma. J. Exp. Med. 183:295-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Signoret, N., J. Oldridge, A. Pelchenmatthews, P. J. Klasse, T. Tran, L. F. Brass, M. M. Rosenkilde, T. W. Schwartz, W. Holmes, W. Dallas, et al. 1997. Phorbol esters and SDF-1 induce rapid endocytosis and down modulation of the chemokine receptor CXCR4. J. Cell Biol. 139:651-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simmons, G., P. R. Clapham, L. Picard, R. E. Offord, M. M. Rosenkilde, T. W. Schwartz, R. Buser, T. N. C. Wells, and A. E. Proudfoot. 1997. Potent inhibition of HIV-1 infectivity in macrophages and lymphocytes by a novel CCR5 antagonist. Science 276:276-279. [DOI] [PubMed] [Google Scholar]
- 40.Tarasova, N. I., R. H. Stauber, and C. J. Michejda. 1998. Spontaneous and ligand-induced trafficking of CXC-chemokine receptor 4. J. Biol. Chem. 273:15883-15886. [DOI] [PubMed] [Google Scholar]
- 41.Torre, V. S., A. J. Marozsan, J. L. Albright, K. R. Collins, O. Hartley, R. E. Offord, M. E. Quinones-Mateu, and E. J. Arts. 2000. Variable sensitivity of CCR5-tropic human immunodeficiency virus type 1 isolates to inhibition by RANTES analogs. J. Virol. 74:4868-4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trkola, A., C. Gordon, J. Matthews, E. Maxwell, T. Ketas, L. Czaplewski, A. E. Proudfoot, and J. P. Moore. 1999. The CC-chemokine RANTES increases the attachment of human immunodeficiency virus type 1 to target cells via glycosaminoglycans and also activates a signal transduction pathway that enhances viral infectivity. J. Virol. 73:6370-6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trkola, A., W. A. Paxton, S. P. Monard, J. A. Hoxie, M. A. Siani, D. A. Thompson, L. Wu, C. R. Mackay, R. Horuk, and J. P. Moore. 1998. Genetic subtype-independent inhibition of human immunodeficiency virus type 1 replication by CC and CXC chemokines. J. Virol. 72:396-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsou, C. L., R. P. Gladue, L. A. Carroll, T. Paradis, J. G. Boyd, R. T. Nelson, K. Neote, and I. F. Charo. 1998. Identification of C-C chemokine receptor 1 (CCR1) as the monocyte hemofiltrate C-C chemokine (HCC)-1 receptor. J. Exp. Med. 188:603-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu, L., G. LaRosa, N. Kassam, C. J. Gordon, H. Heath, N. Ruffing, H. Chen, J. Humblias, M. Samson, M. Parmentier, et al. 1997. Interaction of chemokine receptor CCR5 with its ligands: multiple domains for HIV-1 gp120 binding and a single domain for chemokine binding. J. Exp. Med. 186:1373-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ylisastigui, L., J. Vizzavona, E. Drakopoulou, P. Paindavoine, C.-F. Calvo, M. Parmentier, J. C. Gluckman, C. Vita, and A. Benjouad. 1998. Synthetic full-length and truncated RANTES inhibit HIV-1 infection of primary macrophages. AIDS 12:977-984. [PubMed] [Google Scholar]