Abstract
Bioassay-guided fractionation of the extract from the fermentation broth of Streptomyces spectabilis BCC 4785 led to the isolation of three principle antimalarial agents, metacycloprodigiosin, bafilomycin A1, and spectinabilin. Metacycloprodigiosin exhibited potent in vitro activity against Plasmodium falciparum K1, with a 50% inhibitory concentration of 0.0050 ± 0.0010 μg/ml, while its cytotoxicity was much weaker.
Malaria is a serious endemic disease in many parts of Africa, Asia, Latin America, and Oceania, affecting 5% of the world's population, and mortality is estimated to be over 1 million deaths each year (13, 21). Because of the worsening problems of drug resistance, there has been an urgent need for the discovery of a new chemical class of antimalarial agents (4). As part of an ongoing natural product research program, we have been screening microbial extracts for in vitro antimalarial activity (7-9). Among these, an extract from Streptomyces spectabilis BCC 4785 showed significant activity against Plasmodium falciparum (K1, a multidrug-resistant strain), with a 50% inhibitory concentration (IC50) of 0.01 μg/ml. Therefore, mass fermentation and activity-guided isolation of antimalarial agents from this strain have been undertaken.
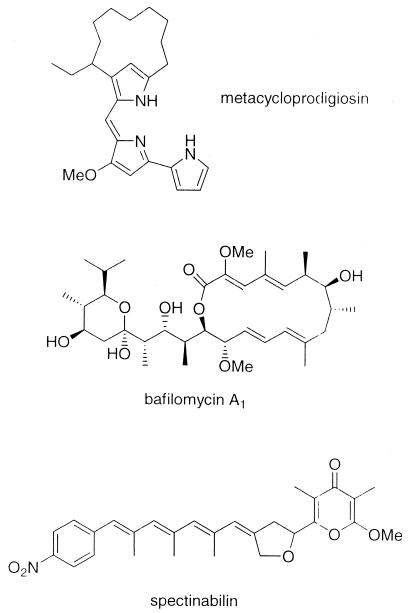
S. spectabilis was isolated from a soil sample collected in Thailand, identified to the species level, and deposited at the BIOTEC Culture Collection as BCC 4785. The strain was incubated in a fermentor containing 75 liters of a liquid medium (10.0 g of soluble starch, 1.0 g of K2HPO4, 1.0 g of MgSO4 · 7H2O, 1.0 g of NaCl, 2.0 g of (NH4)2SO4, and 3.0 g of CaCO3 per liter). Chromatographic separation and purification of the methanolic extract from mycelia led to the isolation of three known compounds, metacycloprodigiosin (free-base form, orange powder, 490 mg; further purified as a hydrochloride, magenta powder) (18, 19), bafilomycin A1 (colorless crystals, 14 mg) (1, 20), and spectinabilin (orange powder, 41 mg) (11). The structures of these compounds (Fig. 1) were identified by spectroscopic analyses and comparison with the literature data.
FIG. 1.
Structures of the metabolites from S. spectabilis BCC 4785.
An assay for activity against P. falciparum K1 was performed by using a standard protocol (10) based on the microculture radioisotope technique described by Desjardins et al. (3). The reported IC50 represents the concentration that causes a 50% reduction of parasite growth, as indicated by the in vitro uptake of [3H]hypoxanthine by P. falciparum. For comparison, the cytotoxicities of the compounds against human epidermoid carcinoma cells (KB cells), human breast cancer cells (BC-1 cells), and African green monkey kidney fibroblasts (Vero cells) were screened by using a colorimetric method (16). The IC50s of the standard compound, ellipticine, in our system were 0.46 μg/ml for KB cells and 0.60 μg/ml for BC-1 cells.
The bioassay results are summarized in Table 1. Metacycloprodigiosin hydrochloride and bafilomycin A1 exhibited significant antimalarial activity, while spectinabilin moderately inhibited the proliferation of P. falciparum K1. The cytotoxic activity of metacycloprodigiosin hydrochloride was much weaker than its antimalarial activity.
TABLE 1.
Antimalarial activities and cytotoxicities of compounds isolated from S. spectabilis BCC 4785
| Compound | Activity (IC50, μg/ml) against P. falciparum K1 | Cytotoxicity (IC50, μg/ml) for:
|
||
|---|---|---|---|---|
| KB cells | BC-1 cells | Vero cells | ||
| Metacycloprodigiosin hydrochloridea | 0.0050 ± 0.0010 | 0.36 ± 0.02 | 0.27 ± 0.01 | 1.35 ± 0.28 |
| Bafilomycin A1a | 0.041 ± 0.010 | 0.27 ± 0.03 | 0.20 ± 0.04 | 1.14 ± 0.04 |
| Spectinabilin | 7.8 | 0.10 | 0.80 | 20 |
| Chloroquine diphosphateb | 0.16 | 16 | >20 | >20 |
| Artemisininb | 0.0011 | >20 | >20 | >20 |
Assays were performed in triplicate for these highly active compounds; values are means and standard deviations.
Standard antimalarial compounds.
Prodigiosins have been known to exhibit a wide range of biological activities, and recent investigations on their immunosuppressive activities (5, 14, 17) and activities as proton pump inhibitors (12, 15) have sparked renewed interest in these tripyrrole pigments. The in vivo activities of metacycloprodigiosin hydrochloride and several other prodigiosins against Plasmodium berghei in mice have been reported (2, 6). According to the literature (6), elongation of the mean survival time of P. berghei-infected mice by oral administration of metacycloprodigiosin hydrochloride was observed with a dose of 20 mg/kg. Although members of this class of compounds have shown activity against malaria in an animal model, little has been done concerning their further development. This situation may be due to the lack of availability of compounds in large amounts. To the best of our knowledge, this is the first report on the in vitro activity of a prodigiosin against a human malaria parasite (P. falciparum). The high antiplasmodial activity, good selectivity index, and structural novelty of this class of compounds deserve further investigation.
Acknowledgments
Financial support from the Biodiversity Research and Training Program (BRT) is gratefully acknowledged. The BIOTEC antimalarial screening laboratory was partly supported by the Thailand-Tropical Diseases Research Programme (T-2). Y.T. thanks BIOTEC, NSTDA, for the senior research fellowship award.
We are grateful to Arinthip Thamchaipenet, Kasetsart University, for identification of strain BCC 4785.
REFERENCES
- 1.Baker, G. H., B. J. Brown, R. J. J. Dorgan, J. R. Everett, S. V. Ley, A. M. Z. Slawin, and D. J. Williams. 1987. A conformational study of bafilomycin A1 by X-ray crystallography and NMR techniques. Tetrahedron Lett. 28:5565-5568. [Google Scholar]
- 2.Castro, A. J. 1967. Antimalarial activity of prodigiosin. Nature 903-904. [DOI] [PubMed]
- 3.Desjardins, R. E., C. J. Canfield, and J. D. Chulay. 1979. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemother. 16:710-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ekthawatchai, S., M. Isaka, P. Kittakoop, P. Kongsaeree, C. Sirichaiwat, M. Tanticharoen, B. Tarnchompoo, Y. Thebtaranonth, and Y. Yutavong. 1999. Synthetic and naturally occurring antimalarials. J. Heterocycl. Chem. 36:1599-1605. [Google Scholar]
- 5.Fürstner, A., J. Grabowski, C. W. Lehmann, T. T. Kataoka, and K. Nagai. 2001. Synthesis and biological evaluation of nonylprodigiosin and macrocyclic prodigiosin analogues. ChemBioChem 2:60-68. [DOI] [PubMed] [Google Scholar]
- 6.Gerber, N. N. 1975. A new prodiginine (prodigiosin-like) pigment from streptomyces. Antimalarial activity of several prodiginines. J. Antibiot. 28:194-199. [DOI] [PubMed] [Google Scholar]
- 7.Isaka, M., A. Jaturapat, W. Kladwang, J. Punya, Y. Lertwerawat, M. Tanticharoen, and Y. Thebtaranonth. 2000. Antiplasmodial compounds from the wood-decayed fungus Xylaria sp. BCC 1067. Planta Med. 66:473-475. [DOI] [PubMed] [Google Scholar]
- 8.Isaka, M., J. Punya, Y. Lertwerawat, M. Tanticharoen, and Y. Thebtaranonth. 1999. Antimalarial activity of macrocyclic trichothecenes isolated from the fungus Myrothecium verrucaria. J. Nat. Prod. 62:329-331. [DOI] [PubMed] [Google Scholar]
- 9.Isaka, M., M. Tanticharoen, P. Kongsaeree, and Y. Thebtaranonth. 2001. Structures of cordpyridones A-D, antimalarial N-hydroxy- and N-methoxy-2-pyridones from the insect pathogenic fungus Cordyceps nipponica. J. Org. Chem. 66:4803-4808. [DOI] [PubMed] [Google Scholar]
- 10.Jaturapat, A., M. Isaka, N. L. Hywel-Jones, Y. Lertwerawat, S. Kamchonwongpaisan, K. Kirtikara, M. Tanticharoen, and Y. Thebtaranonth. 2001. Bioxanthracenes from the insect pathogenic fungus Cordyceps pseudomilitaris BCC 1620. I. Taxonomy, fermentation, isolation and antimalarial activity. J. Antibiot. 54:29-35. [DOI] [PubMed] [Google Scholar]
- 11.Kakinuma, K., C. A. Hanson, and K. L. Rinehart, Jr. 1976. Spectinabilin, a new nitro-containing metabolite isolated from Streptomyces spectabilis. Tetrahedron 32:217-222. [Google Scholar]
- 12.Matsuya, H., M. Okamoto, T. Ochi, A. Nishikawa, S. Shimizu, T. Kataoka, K. Nagai, H. H. Wasserman, and S. Ohkuma. 2000. Reversible and potent uncoupling of hog gastric (H++K+)-ATPase by prodigiosins. Biochem. Pharmacol. 60:1855-1863. [DOI] [PubMed] [Google Scholar]
- 13.Morel, C. M. 2000. Reaching maturity--25 years of the TDR. Parasitol. Today 16:522-526. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura, A., K. Nagai, K. Ando, and G. Tamura. 1986. Selective suppression by prodigiosin of the mitogenic response of murine splenocytes. J. Antibiot. 39:1155-1159. [DOI] [PubMed] [Google Scholar]
- 15.Sato, T., H. Konno, Y. Tanaka, T. Kataoka, K. Nagai, H. H. Wasserman, and S. Ohkuma. 1998. Prodigiosins as a new group of H+/Cl− symporters that uncouple proton translocators. J. Biol. Chem. 273:21455-21462. [DOI] [PubMed] [Google Scholar]
- 16.Skehan, P., R. Storeng, D. Scudiero, A. Monks, J. McMahon, D. Vistica, J. T. Warren, H. Bokesch, S. Kenney, and M. R. Boyd. 1990. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 82:1107-1112. [DOI] [PubMed] [Google Scholar]
- 17.Songia, S., A. Mortellaro, S. Taverna, C. Fornasiero, E. A. Scheiber, E. Erba, F. Colotta, A. Mantovani, A. M. Isetta, and J. Golay. 1997. Characterization of the new immunosuppressive drug undecylprodigiosin in human lymphocytes: retinoblastoma protein, cyclin-dependent kinase-2, and cyclin-dependent kinase-4 as molecular targets. J. Immunol. 158:3987-3995. [PubMed] [Google Scholar]
- 18.Wasserman, H. H., D. D. Keith, and G. C. Rodgers. 1976. The structure of metacycloprodigiosin. Tetrahedron 32:1855-1861. [Google Scholar]
- 19.Wasserman, H. H., G. C. Rodgers, and D. D. Keith. 1969. Metacycloprodigiosin, a tripyrrole pigment from Streptomyces longisporus ruber. J. Am. Chem. Soc. 91:1263-1264. [DOI] [PubMed] [Google Scholar]
- 20.Werner, G., H. Hagenmaier, K. Albert, and H. Kohlshorn. 1983. The structure of the bafilomycins, a new group of macrolide antibiotics. Tetrahedron Lett. 24:5193-5196. [Google Scholar]
- 21.World Health Organization. 1998. W. H. O. fact sheet 94. World Health Organization, Geneva, Switzerland.