Abstract
Recrudescences were simulated in vitro with drug treatment to examine how drug-sensitive parasites survive the treatment. Various numbers of cultured parasites were treated with lethal doses of pyrimethamine or mefloquine for various lengths of time. Recrudescences were observed in parasite populations with larger initial numbers of parasites when the treatment duration was prolonged. Equal numbers of parasitized erythrocytes were treated with various concentrations of pyrimethamine or mefloquine. There was no clear linear relationship between the incidence of recrudescence and the drug concentration. Parasites that had recrudesced were continuously allowed to recrudesce in the succeeding recrudescence experiments. Both the duration from the cessation of treatment to the time at which the recrudescent parasitemia level reached 1% and the growth rate of recrudescent parasites were equal among these recrudescences. The recrudescent parasites in these experiments were as sensitive to the drugs as the parasites tested before treatment were. These results suggest that a parasite culture may contain parasites in some phases that are not killed by drug for up to 10 days, which explains the recrudescences that occur even after treatment.
Malaria remains one of the most serious threats to human beings in the world. Every year 300 million to 500 million people are infected with malaria parasites and 1 million people die of malaria (17). The appearance and expansion of drug-resistant Plasmodium falciparum make the chemotherapy of malaria more difficult. Recrudescence is a sign of treatment failure and has been thought to occur due to the selection of drug-resistant parasites. However, there have been reports of discrepancies between clinical findings and laboratory test results; that is, recrudescences occurred after drug treatment, but the recrudescent parasites were sensitive in laboratory drug sensitivity tests (2, 3, 4, 9, 10). Drug-sensitive parasites sometimes recrudesced despite adequate drug treatment (16). The accepted explanation for such a phenomenon was either that the concentration of the drug in plasma was insufficient to suppress parasites or that the concentration was not maintained above the effective value long enough to eliminate the parasites (5, 11, 12).
In a previous study we used an in vitro culture to demonstrate recrudescences after d-sorbitol and pyrimethamine treatment in culture (6). The culture was free of host factors, such as variabilities in pharmacodynamics and pharmacokinetics, specific immunity against the parasites, and possible hiding places for the parasites. It was suggested that the dormant parasites survived the treatments. However, the behaviors of the parasites that recrudesced remain to be clarified. We therefore attempted to examine the frequency of recrudescence and the relationship between recrudescence and drug concentration.
We show in this paper that the incidence of recrudescence in vitro is not related to the drug concentration at which the drug was predicted to eradicate the parasites but is dependent both on the length of treatment duration and on the initial number of parasites. The proportions of parasites in the initial parasite population that recrudesced might be invariable.
MATERIALS AND METHODS
Parasite culture and cloning.
The FCR-3 strain of P. falciparum parasites was maintained in standard medium (13) in a 5% CO2-5% O2-90% N2 atmosphere at 37°C. The medium was changed daily, and the level of parasitemia was counted by Giemsa staining of thin blood films.
Parasite clones were obtained by the limiting dilution method. A ring-form-infected erythrocyte suspension containing 2 parasites per ml at 2% hematocrit was dispensed into a 96-well culture plate at 100 μl per well. On day 6, the blood in the 96-well plate was transferred to 24-well culture plates and supplemented with 400 μl of fresh erythrocyte suspension at 2% hematocrit per well. Four hundred microliters of the fresh erythrocyte suspension was added to each well on days 12 and 18. The level of parasitemia was examined on day 20 and day 24. Three wells were selected on the basis of the parasitemia levels on day 20, and the parasitized erythrocytes from these wells were subjected to two other rounds of the same cloning procedures. The rates of positivity for wells in the first, second, and third rounds of cloning were 11.5, 13.3, and 14.2%, respectively.
Pyrimethamine and mefloquine sensitivity tests.
Mefloquine (Mepha, Aesch- Basel, Switzerland) was dissolved in pure water at a concentration of 10−3 M and was stored at 4°C for 2 weeks. Pyrimethamine (Sigma, St. Louis, Mo.) was dissolved in 0.5% lactic acid (8) at a concentration of 10−2 M and was stored at 4°C for 2 weeks. All recrudescent parasites in this study were examined for their sensitivities to drug treatment when the level of recrudescent parasitemia was more than 2%. Briefly, 0.25 ml of a 20% suspension of parasitized erythrocytes was mixed with aliquots of serial 10-fold dilutions from 2 × 10−6 to 2 × 10−10 M pyrimethamine or mefloquine in standard medium in a 24-well tissue culture plate (final suspension, 10%; final drug concentration, a serial 10-fold dilution from 1 × 10−10 to 1 × 10−6 M). As a control, parasitized erythrocytes were incubated with the medium. On days 2 and 3 of incubation, the drug-containing medium was changed and thin blood films were made. Growth inhibition is presented as the percent parasitemia at each concentration of drug on day 3 compared to the percent parasitemia without drug on the same day. The 50% inhibitory concentration (IC50) of FCR-3 was determined on the basis of the results of sensitivity tests, in which the parasites were treated with serial twofold dilutions of drug covering a range from 10−9 M to 10−7 M. The IC50s of mefloquine and pyrimethamine were 4 × 10−9 and 6 × 10−9 M, respectively.
Examination of the proportion of recrudescent parasites.
An aliquot of the erythrocytes containing 109 parasitized erythrocytes was obtained from the culture with 5% parasitemia. The aliquot was diluted with standard medium to yield 108 parasitized erythrocytes per ml. The cell suspension was diluted with a fresh erythrocyte suspension in standard medium at 10% hematocrit to make 10-fold serial dilutions of parasitized erythrocytes from 107 to 104 per ml. The aliquot containing 109 parasitized erythrocytes was resuspended in 20 ml of drug-containing medium and inoculated into a 200-ml culture flask. Aliquots containing 108, 107, 106, 105, and 104 parasitized erythrocytes were resuspended in 5 ml of drug-containing medium, and the suspension was inoculated into 20-ml culture flask. The drug-containing medium was changed every day. On the day when treatment ceased, the cultures were washed extensively with standard medium, supplemented with 0.1 ml of packed fresh erythrocytes, and inoculated into fresh flasks. The level of parasitemia was examined daily from 7 days after the end of treatment. One-tenth and 0.2 ml of the packed fresh erythrocytes were added to a 20-ml culture flask and a 200-ml culture flask, respectively, every 7 days after treatment until parasites reappeared.
Examination of relationship between recrudescence and drug concentration.
Equal numbers of parasitized erythrocytes were treated with 10-fold serial dilutions of pyrimethamine (from 10−9 to 10−4 M) or mefloquine (from 10−9 to 10−5 M) for 4 days with daily changes of the drug-containing medium. After treatment, the parasitized erythrocytes were washed, supplemented with fresh erythrocytes, and maintained in the same way as described above. The time from the cessation of treatment to the day on which recrudescent parasitemia levels reached or surpassed 1% was recorded.
Comparison of recrudescences in parasites treated with 10−6 and 10−5 M pyrimethamine.
Erythrocytes parasitized with clone FCR-3 were diluted with fresh erythrocytes to obtain 107, 106, 105, and 104 parasitized erythrocytes. They were then treated with 10−6 or 10−5 M pyrimethamine for 4 days. After treatment, they were washed, supplemented with fresh erythrocytes, and inoculated into standard medium in the same way as described above.
Repetition of recrudescence in cloned parasites.
Twenty million parasitized erythrocytes of clone FCR-3 were treated with 10−6 M pyrimethamine for 4 days, washed, supplemented with fresh erythrocytes, and returned to standard medium. The recrudescent parasites at a parasitemia level of more than 2% were again treated in the same way as in the first recrudescence experiment. In this way recrudescences were allowed to occur four more times. The duration from the cessation of treatment to the time at which the recrudescent parasitemia level reached 1% and parasite growth were compared among six recrudescence experiments.
Measurement of residual drug levels.
Six hundred million normal erythrocytes were incubated with standard medium containing pyrimethamine or mefloquine at 10−8, 10−6, and 10−4 M for 4 days with daily changes of the drug-containing medium. As a control, erythrocytes were incubated with standard medium. On day 4, erythrocytes were washed four times with standard medium and lysed by subjecting them to repeated changes of osmotic pressure. Erythrocyte membranes were washed extensively with phosphate-buffered saline.
Six hundred million parasitized erythrocytes at 10% parasitemia were incubated with standard medium containing pyrimethamine at 10−8, 10−6, and 10−4 M and standard medium alone as a control. To harvest the membranes and the water-insoluble sediment, these cells were treated in the same way as the normal erythrocytes.
The sediments were suspended in 200 μl of methanol, mixed vigorously, and centrifuged at 1,800 × g for 5 min at 10°C. The supernatants were analyzed with a high-performance liquid chromatography (HPLC) system (Hitachi, Tokyo, Japan). The solvent for mefloquine detection was a mixture of phosphate-buffered 50 mM sodium sulfate (pH 2.84) and acetonitrile (67:33; vol/vol). The solvent for pyrimethamine detection was a mixture of phosphate-buffered 37.5 mM sodium sulfate (pH 2.84) and acetonitrile (3:1; vol/vol). The flow rate was 1.0 ml/min. The limits of detection for mefloquine and pyrimethamine were less than 10−7 M. The peak area ratios in the calibration standard curves for mefloquine and pyrimethamine were linear over the range from 10−6 to 10−4 M, respectively.
Statistical analysis.
Variations in the duration from the cessation of treatment to 1% parasitemia among the various treatments tested in the study were analyzed by two-way analysis of variance, while multiple comparisons of means were done by use of the Bonferroni method. Statistical calculations were conducted with Systat statistical software.
RESULTS
Proportion of parasites that recrudesced.
To evaluate the number of parasites that survived treatment, serial dilutions of parasitized erythrocytes were exposed to 10−6 M pyrimethamine treatment for various lengths of time. No recrudescence was observed for 104, 105, 107, and 108 parasitized erythrocytes for 2 months after 4, 6, 8, and 10 days of pyrimethamine treatment, respectively (Table 1). In contrast, recrudescence occurred in 105 and 106 parasitized erythrocytes after 4 days of treatment. With a longer duration of treatment, larger numbers of parasitized erythrocytes were needed to observe recrudescence.
TABLE 1.
Recrudescences after pyrimethamine treatment
| Treatment duration (days) | Recrudescence for the following initial no. of parasitized erythrocytesa:
|
|||||
|---|---|---|---|---|---|---|
| 104 | 105 | 106 | 107 | 108 | 109 | |
| 4 | − | ++ | ++ | ND | ND | ND |
| 6 | ND | − | ++ | ++ | ND | ND |
| 8 | ND | ND | − | − | + | ++ |
| 10 | ND | ND | ND | − | − | + |
The indicated numbers of parasitized erythrocytes were exposed to 10−6 M pyrimethamine for the indicated numbers of days, washed, and returned to standard medium. Experiments were carried out in triplicate. −, no recrudescences were observed for 2 months after treatment or recrudescence was observed in one of three flasks; +, recrudescences were observed in two of three flasks or in all three flasks; ++, recrudescences were observed in all three flasks; ND, not done. The same experiments were repeated six times, and the accumulated data are presented.
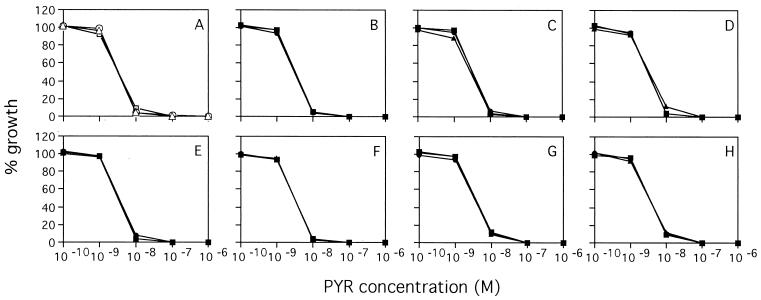
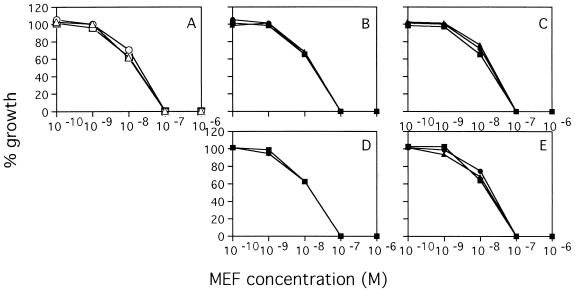
No recrudescence was observed in 105 and 106 parasitized erythrocytes for 2 months after 4 and 6 days of treatment with 10−6 M mefloquine, respectively (Table 2). Recrudescence occurred in 106 and 107 parasitized erythrocytes after 4 days of mefloquine treatment and 107 and 108 parasitized erythrocytes after 6 days of treatment. However, recrudescences did not consistently occur after 8 days of mefloquine treatment due to heavy hemolysis. All recrudescent parasites were as sensitive to pyrimethamine and mefloquine as the parasites tested before treatment were (Fig. 1 and 2).
TABLE 2.
Recrudescences after mefloquine treatment
| Treatment duration (days) | Recrudescence for the following initial no. of parasitized erythrocytesa:
|
|||||
|---|---|---|---|---|---|---|
| 104 | 105 | 106 | 107 | 108 | 109 | |
| 4 | − | − | ++ | ++ | ND | ND |
| 6 | ND | − | − | + | ++ | ND |
| 8 | ND | ND | − | − | − | − |
The indicated numbers of parasitized erythrocytes were treated with 10−6 M mefloquine for the indicated numbers of days, washed, and returned to standard medium. The same experiments were repeated five times, and the accumulated data are presented. The definitions of −, +, ++, and ND are as described in footnote a of Table 1.
FIG. 1.
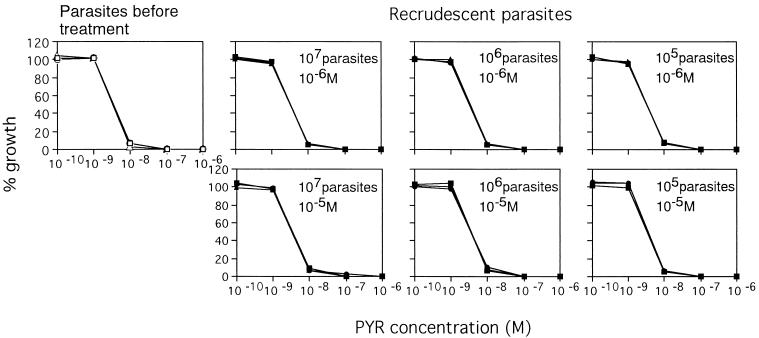
Recrudescent parasites showed the same patterns of sensitivity to pyrimethamine (PYR) as the control parasites did. (A) Control parasites; (B to H) recrudescent parasites: 105 for 4 days (B), 106 for 4 days (C), 106 for 6 days (D), 107 for 6 days (E), 108 for 8 days (F), 109 for 8 days (G), and 109 for 10 days (H).
FIG. 2.
Recrudescent parasites showed the same pattern of sensitivity to mefloquine (MEF) as the control parasites did. (A) Control parasites; (B to E) recrudescent parasites: 106 for 4 days (B), 107 for 4 days (C), 107 for 6 days (D), 108 for 6 days (E).
Relationship between recrudescence and drug concentration.
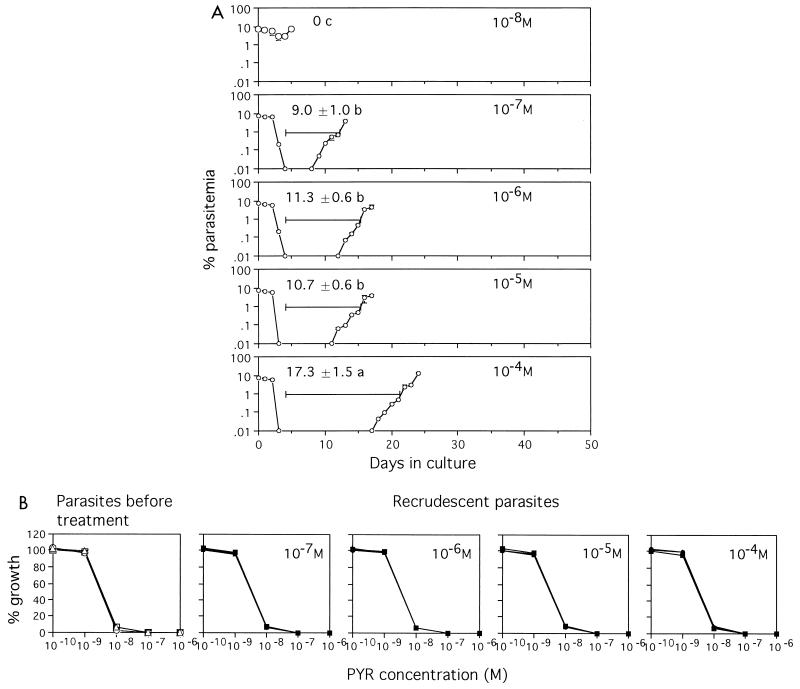
Treatment with 10−8 M pyrimethamine resulted in little decrease in the level of parasitemia, which subsequently increased again after treatment (Fig. 3A). Treatment with 10−7, 10−6, 10−5, and 10−4 M pyrimethamine reduced the parasitemias to undetectable levels during treatment. While parasites reappeared in the parasitized erythrocytes treated with 10−7, 10−6, and 10−5 M pyrimethamine several days after treatment, the reappearance of parasites was delayed in those treated with 10−4 M pyrimethamine. There was no significant difference (P = 0.744) among the durations from the cessation of treatment to 1% parasitemia observed with treatment with 10−7, 10−6, and 10−5 M pyrimethamine. Recrudescent parasites were as sensitive to pyrimethamine as parasites tested before treatment were (Fig. 3B).
FIG. 3.
(A) Parasites recrudesce independently of the pyrimethamine concentration. Fifty million parasitized erythrocytes (7% parasitemia) were exposed to the indicated concentrations of pyrimethamine for 4 days, washed, and returned to standard medium. Experiments were carried out in triplicate. Mean ± standard deviation levels of parasitemia and mean ± standard deviation durations from the day when treatment ceased until the day when the recrudescent parasitemia level reached or surpassed 1% are shown. The data are representative of three separate experiments. Means followed by the same letter were not significantly different (P = 0.744). (B) Recrudescent parasites showed the same pattern of sensitivity to pyrimethamine (PYR) as the parasites tested before treatment did.
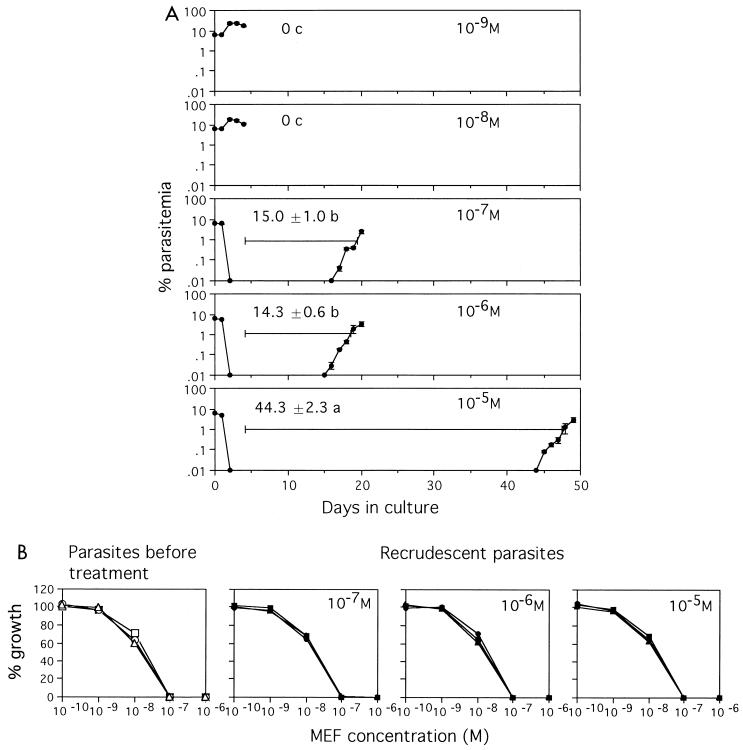
As mefloquine caused stronger hemolysis at higher concentrations, a concentration of 10−4 M could not be used in this experiment. With treatment with 10−9 and 10−8 M mefloquine, there was little decrease in the level of parasitemia, which subsequently increased after treatment (Fig. 4A). For parasites exposed to 10−7 and 10−6 M mefloquine, the durations from the discontinuation of treatment to 1% parasitemia were equal (P = 0.565). The reappearance of parasites was markedly delayed in those treated with 10−5 M mefloquine. Recrudescent parasites were as sensitive to mefloquine as the parasites tested before treatment were (Fig. 4B).
FIG. 4.
(A) Parasites recrudesce independently of the mefloquine concentration. Fifty million parasitized erythrocytes (6.5% parasitemia) were treated with the indicated concentrations of mefloquine. Experiments were carried out in triplicate. Mean ± standard deviation levels of parasitemia and mean ± standard deviation durations from the day when treatment ceased until the day when the recrudescent parasitemia level reached or surpassed 1% are shown. Data shown are representative of three separate experiments. Means followed by the same letter were not significantly different (P = 0.565). (B) Recrudescent parasites showed the same pattern of sensitivity to mefloquine (MEF) as the parasites tested before treatment did.
Measurement of residual pyrimethamine and mefloquine levels by HPLC.
As shown in Tables 1 and 2, the proportions of parasites that recrudesced after treatment with 10−6 M pyrimethamine were higher than the proportions that recrudesced after treatment with 10−6 M mefloquine. The durations from the day when treatment ceased until the day when the recrudescent parasitemia level reached or surpassed 1% observed after treatment with 10−7, 10−6, and 10−5 M pyrimethamine were shorter than the durations observed after treatment with similar concentrations of mefloquine, as shown in Fig. 3A and 4A. Even in the experiments with pyrimethamine, the duration observed after treatment with 10−4M pyrimethamine was longer than those seen after treatment with 10−7, 10−6, and 10−5 M pyrimethamine. It is likely that the drugs remained even after treatment and that they affected the parasites. The levels of residual pyrimethamine and mefloquine in the cultures were examined to test this possibility.
No mefloquine was detected in the membrane fractions of normal erythrocytes which had been treated with 10−8 M mefloquine for 4 days or those that had been incubated in standard medium. On the other hand, mefloquine remained in the erythrocytes treated with 10−6 or 10−4 M (7.28 and 762 ng/mg of protein, respectively). Pyrimethamine was not detected in normal erythrocytes, which had been treated with the same concentrations of pyrimethamine as mefloquine (Table 3). These data indicated that mefloquine remained in culture by binding to the cell membrane. Chevli and Fitch (1) showed that mefloquine had a high affinity for phospholipids in the erythrocyte membrane.
TABLE 3.
Detection of residual pyrimethamine both in erythrocyte membranes and in the insoluble fractions from parasitized erythrocytesa
| Samples | Treatment (M) | Amt detected (μg/whole sample) |
|---|---|---|
| Normal erythrocytes | Standard medium | NDb |
| 10−8 | ND | |
| 10−6 | ND | |
| 10−4 | ND | |
| Infected erythrocytes | Standard medium | ND |
| 10−8 | ND | |
| 10−6 | ND | |
| 10−4 | 7.10 |
Membrane and water-insoluble fraction were collected from normal erythrocytes and parasitized erythrocytes treated with the indicated concentrations of pyrimethamine for 4 days with changes in osmotic pressure and extensive washing with P phosphate-buffered saline. Residual pyrimethamine was measured by HPLC.
ND, not detected.
Pyrimethamine was not detected in the sediment fraction of infected erythrocytes treated with 10−8 or 10−6 M pyrimethamine. On the other hand, pyrimethamine remained in infected erythrocytes treated with 10−4 M pyrimethamine.
Effect of residual mefloquine on parasite growth.
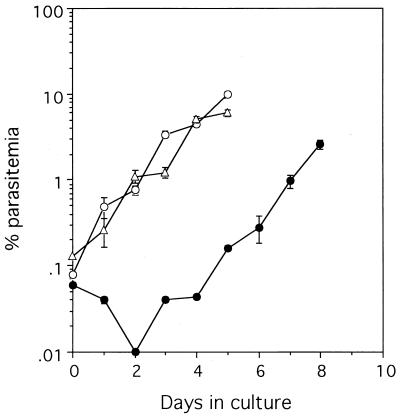
Parasites were cultured in erythrocytes treated with mefloquine to examine whether the residual mefloquine affected the multiplication of parasites. The level of parasitemia decreased for the first 2 days and then increased when the parasites were cultured in erythrocytes that had been treated with 10−6 M mefloquine for 4 days and washed with standard medium (Fig. 5). After being treated and washed as described above, the erythrocytes which were reincubated for a week in standard medium with daily medium changes supported parasite growth in the same manner that normal erythrocytes did.
FIG. 5.
Residual mefloquine suppressed parasite growth, and its effect was reduced during the incubation. Parasitized erythrocytes at 18% parasitemia were diluted with fresh erythrocytes that had been treated as follows: by treatment with 10−6 M mefloquine for 4 days and washing (filled circles); by treatment with mefloquine, washing, and incubation in standard medium for 7 days (open triangles); or by incubation in standard medium (open circles). Parasite culture was carried out in triplicate. Mean ± standard deviation levels of parasitemia are shown. The data are representative of two separate experiments.
Comparison of recrudescences with different concentrations of pyrimethamine.
The mode of recrudescence was compared by the use of pyrimethamine at concentrations between 10−5 and 10−6 M and different numbers of cloned parasites. The durations were equal (P = 0.812) between pyrimethamine concentrations of 10−5 and 10−6 M for 107, 106, and 105 parasitized erythrocytes (Table 4). Ten thousand parasitized erythrocytes showed no recrudescence during a period of 2 months.
TABLE 4.
Duration from cessation of treatment to 1% after treatment with parasitemia between 10−6 and 10−5 M pyrimethaminea
| Initial no. of parasitized erythrocytes | Duration (days) for the following pyrimethamine concn (M):
|
|
|---|---|---|
| 10−6 | 10−5 | |
| 104 | No recrudescence | No recrudescence |
| 105 | 18.0 ± 1.0 | 18.3 ± 1.2 |
| 106 | 14.3 ± 1.5 | 13.6 ± 0.6 |
| 107 | 11.7 ± 0.6 | 12.3 ± 0.6 |
The indicated numbers of parasitized erythrocytes of clone FCR-3 were treated with 10−6 or 10−5 M pyrimethamine for 4 days, washed, and maintained in standard medium. Experiments were carried out in triplicate and were repeated three times. Mean ± standard deviation durations from a representative experiment are shown. The difference in the mean duration between initial parasitized erythrocyte numbers was significant (P < 0.001). The difference in the mean duration between the two concentrations was not statistically significant (P = 0.812).
All recrudescent parasites were as sensitive to pyrimethamine as the parasites tested before treatment were (Fig. 6).
FIG. 6.
Recrudescent parasites showed the same pattern of sensitivity to pyrimethamine (PYR) as parasites tested before treatment did.
Repeated recrudescences.
Cloned parasites were recrudesced repeatedly six times, and the duration from the end of treatment until the detection of 1% parasitemia was recorded (Table 5). The mean duration was 11.8 days. There was no significant difference (P = 0.476) among the durations for the six recrudescences. The mean length of time from the day that the level of parasitemia for recrudescent parasites exceeded 0.01% until the day that the level of parasitemia reached or surpassed 1% was 4.4 days. There was no significant difference (P = 0.923) in the growth periods for the six recrudescences. Recrudescent parasites had the same level of sensitivity to pyrimethamine as clone FCR-3 in repeated recrudescence experiments, similar to the results shown in Fig. 6.
TABLE 5.
Duration from cessation of treatment to 1% parasitemia and growth periods from 0.01 to 1% parasitemia in a series of six successive recrudescencesa
| Recrudescence | Duration (days) | Growth period (days) |
|---|---|---|
| First | 11.6 ± 0.6 | 4.3 ± 0.6 |
| Second | 12.0 ± 0.0 | 4.7 ± 0.6 |
| Third | 11.3 ± 1.5 | 4.3 ± 0.6 |
| Fourth | 11.0 ± 1.0 | 4.3 ± 0.6 |
| Fifth | 12.3 ± 1.5 | 4.7 ± 0.6 |
| Sixth | 12.7 ± 1.2 | 4.3 ± 0.6 |
The recrudescence experiment was repeated successively six times by using cloned parasites. Each recrudescence experiment was carried out in triplicate, and the recrudescent parasites in three flasks were mixed and subjected to the following recrudescence experiment. A series of recrudescence experiments was conducted three times. The mean± standard deviation duration and growth period in a representative series are shown with SD. The difference in the mean duration was not statistically significant (P = 0.476). The difference in the mean growth period was not statistically significant (P = 0.923).
DISCUSSION
Recrudescences were produced in cultures. The recrudescent parasites in these recrudescence experiments were as sensitive to drugs as the parasites tested before treatment, as shown in Fig. 1, 2, 3B, 4B, and 6. The results suggest that drug-sensitive parasites survived drug treatment for several days in culture.
No parasites in culture inoculated with 104, 105, 107, and 108 parasites survived pyrimethamine treatment for 4, 6, 8, and 10 days, respectively, as shown in Table 1. Despite the use of the same treatment for the same periods, recrudescences occurred in the cultures that contained 10 times more parasites than those amounts mentioned above. A simple explanation for this result may be incomplete eradication. However, parasites seemed to have survived drug treatment for several days by escaping the effect of the drug. Assuming that treatment with pyrimethamine at 10−6 M reduces the number of parasites 100-fold per one cycle (killing rate, 100), treatment for 4, 6, 8, and 10 days eliminated parasites 104-, 106-, 108-, and 1010-fold, respectively. Recrudescence may occur in 105, 107, and 109 parasites after treatment for 4, 6, and 8 days, respectively. These results are comparable to those seen in Table 1. As shown in Fig. 3A and Table 4, recrudescence occurred independently of the pyrimethamine concentration at concentrations of 10−6 and 10−5 M. Besides, the killing rate obtained microscopically with pyrimethamine at both 10−6 and 10−5 M is actually 1,000 (range, 870 to 1,330) per one cycle in our hands. It is not likely that recrudescence arose because of a low killing rate.
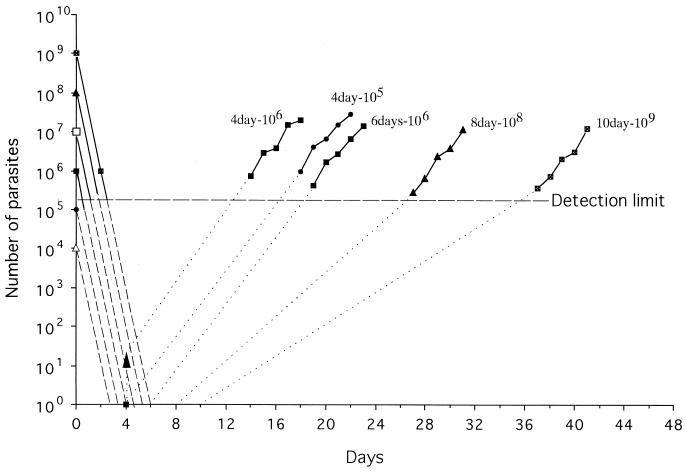
To analyze the relationship between killing of parasites by treatment and growth of surviving parasites, we have replotted the data for the logarithmic kill and growth phases from Table 1, as described by White (15) (Fig. 7). If the killing rate remains constant with succeeding cycles (15), it is predicted that treatment with pyrimethamine at 10−6 M for 4 days will completely kill 104 and 105 parasites (Fig. 7). Similarly, 6 days of treatment is enough to eradicate up to 108 parasites. A total of 109 parasites are eradicated far before the end of treatment for 8 and 10 days. This analysis suggests that the experimental treatments were adequate to eradicate the parasites and that the parasites recrudesced despite the adequate treatment.
FIG. 7.
Killing by pyrimethamine treatment and growth of recrudescent parasites. The figure was prepared with the data from Table 1. All pyrimethamine treatment durations except the 6-day treatment against 106 parasites eradicated within the treatment duration. Broken lines, killing rate if the killing rate remains constant with succeeding cycles; dotted lines, numbers of surviving parasites extrapolated from a growth rate above the detection limit.
The numbers of surviving parasites are extrapolated from a growth rate above the detection limit (Fig. 7). One in 105 and about 10 in 106 parasites might be alive at the end of 4 days of treatment. The growth rates of parasites below and above the detection limit are similar between parasites treated for 4 days and those treated for 6 days. The observed growth rates for parasites treated for 8 and 10 days are parallel to those for parasites treated for 4 days, but the level of growth below the detection limit for parasites treated for 8 and 10 days seems much slower than that for those treated for 4 days. These results suggest that the commencement of intraerythrocyte development was delayed or that the rate of growth of the parasites slowed. It is likely that multiplication of parasites might be retarded after exposure of the parasites to drug for a few days or that parasites might not enter the growth phase during drug treatment.
Wernsdorfer (14) referred to the possibility that parasites stop passing through their cell cycles in order to repair the damage induced by drug treatment. If recrudescence occurred by this mechanism, treatment with a higher concentration of drug might cause damage in a larger number of parasites. Those damaged parasites might then repair the damage by spending various lengths of time without undergoing cell cycles. As shown in Table 4, the durations from the time of cessation of treatment to 1% parasitemia were equal for the 10−6 and 10−5 M treatments, and no recrudescences arose in assays with 104 parasitized erythrocytes. Drug treatment might not be likely to induce parasites to stop passing through the cell cycle in order to undergo repair.
It is also likely that drug-sensitive parasites escape the effects of drugs by remaining in the ring-form stage during the treatment period. Ring-form stages of parasites are not susceptible to drug treatment. In a population of parasites there may be parasites with various lengths of ring-form stages. As indicated in Table 1, a larger population shows a larger variation in the length of the ring-form stage. This may be the reason that recrudescence does not depend on the drug concentration (Fig. 3A and Table 4) but does depend on the initial number of parasites and the duration of treatment. Differences in the lengths of the ring-form stage may be produced at the merozoite stage, as different rhoptry proteins are expressed in merozoites originating from a single schizont (7).
Recrudescences occurred in the same manner in six consecutive recrudescence experiments with cloned parasites, as shown in Table 5. These results suggest that the proportion of inactive parasites in a parasite population may be unchanged in each experiment or that similar numbers of parasites in each recrudescence experiment may slow their growth after exposure to drug.
Treatment with mefloquine at 10−6 M reduces the number of parasites more than 200-fold per one cycle. As shown in Fig. 5, the residual mefloquine in uninfected erythrocytes delayed parasite growth by about 4 days. Residual mefloquine reduces the number of parasites 100-fold even after treatment. If the killing rate is constant, as is assumed for pyrimethamine treatment, in our study the mefloquine treatment was adequate to eradicate parasites. Despite the adequate mefloquine treatment, recrudescence was observed in 106 parasites treated for 4 days and in 107 parasites treated for 6 days, as shown in Table 2. It is likely that the recrudescence that occurred after mefloquine treatment was caused by parasites in a lag phase or parasites in an inactive ring form, as was conceived to be the cause of recrudescence after pyrimethamine treatment.
Although the killing and growth of parasites that occur below the detectable levels of parasitemia remain to be verified, this study provides evidence that drug-sensitive parasites that escape the effect of a drug may cause recrudescence.
Acknowledgments
We thank Gloria Lumbera Enriquez for kind help.
This work was partially supported by a grant-in-aid for Scientific Research on Priority Areas from the Ministry of Education, Science, Sports and Culture of Japan.
REFERENCES
- 1.Chevli, R., and C. D. Fitch. 1982. The antimalarial drug mefloquine binds to membrane phospholipids. Antimicrob. Agents Chemother. 21:581-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kean, B. H. 1979. Chloroquine resistant falciparum malaria from Africa. JAMA 241:395.. [PubMed] [Google Scholar]
- 3.Lemnge, M. M., and A. W. Inambao. 1988. In vitro and in vivo sensitivity of Plasmodium falciparum to chloroquine at Lubwe and Kalene in Zambia: use of amodiaquine as an alternative antimalarial drug. Trans. R. Soc. Trop. Med. Hyg. 82:194-196. [DOI] [PubMed] [Google Scholar]
- 4.Looareesuwan, S., D. E. Kyle, C. Viravan, S. Vanijanonta, P. Wilairatana, P. Charoenlarp, C. J. Canfield, and H. K. Webster. 1992. Treatment of patients with recrudescent falciparum malaria with a sequential combination of artesunate and mefloquine. Am. J. Trop. Med. Hyg. 47:99-108. [DOI] [PubMed] [Google Scholar]
- 5.Looareesuwan, S. 1994. Overview of clinical studies on artemisinin derivatives in Thailand. Trans. R. Soc. Trop. Med. Hyg. 88(Suppl.):9-11. [DOI] [PubMed] [Google Scholar]
- 6.Nakazawa, S., H. Kanbara, and M. Aikawa. 1995. Plasmodium falciparum: recrudescence of parasites in culture. Exp. Parasitol. 81:556-563. [DOI] [PubMed] [Google Scholar]
- 7.Preiser, P. R., W. Jarra, T. Capiod, and G. Snounou. 1999. A rhoptry-protein-associated mechanism of clonal phenotypic variation in rodent malaria. Nature 398:618-622. [DOI] [PubMed] [Google Scholar]
- 8.Richards, W. H. G., and B. K. Maples. 1979. Studies on Plasmodium falciparum in continuous cultivation. I. The effect of chloroquine and pyrimethamine on parasite growth and viability. Ann. Trop. Med. Parasitol. 73:99-108. [PubMed] [Google Scholar]
- 9.Ringwald, P., and L. K. Basco. 1999. Comparison of in vivo and in vitro tests of resistance in patients treated with chloroquine in Younde, Cameroon. Bull. W. H. O. 77:34-43. [PMC free article] [PubMed] [Google Scholar]
- 10.Schwartz, I. K., D. Payne, C. C. Campbell, and O. J. Khatib. 1983. In-vivo and in-vitro assessment of chloroquine-resistant Plasmodium falciparum malaria in Zanzibar. Lancet i:1003-1005. [DOI] [PubMed]
- 11.Slutsker, L. M., C. O. Khoromana, D. Payne, C. R. Allen, J. J. Worima, D. L. Heymann, L. Patchen, and R. W. Steketee. 1990. Mefloquine therapy for Plasmodium falciparum malaria in children under 5 years of age in Malawi: in vivo/in vitro efficacy and correlation of drug concentration with parasitological outcome. Bull. W. H. O. 68:53-59. [PMC free article] [PubMed] [Google Scholar]
- 12.Ter Kuile, F. O., C. Luxemburger, F. Nosten, K. L. Thwai, T. Chongsuphajaisiddhi, and N. J. White. 1995. Predictors of mefloquine treatment failure: a prospective study of 1590 patients with uncomplicated falciparum malaria. Trans. R. Trop. Med. Hyg. 89:660-664. [DOI] [PubMed] [Google Scholar]
- 13.Trager, W., and J. B. Jensen. 1976. Human malaria parasites in continuous culture. Science 193:673-675. [DOI] [PubMed] [Google Scholar]
- 14.Wernsdorfer, W. H. 1994. Epidemiology of drug resistance in malaria. Acta Trop. 56:143-156. [DOI] [PubMed] [Google Scholar]
- 15.White, N. J. 1997. Assessment of the pharmacodynamic properties of antimalarial drugs in vivo. Antimicrob. Agents Chemother. 41:1413-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White, N. J. 1998. Why is it that antimalarial drug treatments do not always work? Ann. Trop. Med. Parasitol. 92:449-458. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. 1997. World malaria situation in 1994. Wkly. Epidemiol. Rec. 72:269-276.