Abstract
Osmotically stabilized Escherichia coli cells subjected to freezing and thawing were utilized as the source of enzymes for a peptidoglycan pathway assay that can be used to simultaneously test all targets of the committed steps of cell wall biosynthesis. The use of 14C-labeled UDP-N-acetylglucosamine (UDP-GlcNAc) as a substrate allows the direct detection of cross-linked peptidoglycan formed. The assay was validated with known antibiotics. Fosfomycin was the strongest inhibitor of the pathway assay, with a 50% inhibitory concentration of 1 μM. Flavomycin, bacitracin, vancomycin, d-cycloserine, penicillin G, and ampicillin also inhibited formation of radiolabeled peptidoglycan by the E. coli cells. Screening of compounds identified two inhibitors of the pathway, Cpd1 and Cpd2. Subsequent tests with a biochemical assay utilizing purified enzyme implicated UDP-GlcNAc enolpyruvyl transferase (MurA) as the target of Cpd1. This compound inhibits the first enzyme of the pathway in a time-dependent manner. Moreover, enzyme inactivation is dependent on preincubation in the presence of UDP-GlcNAc, which forms a complex with MurA, exposing its active site. Cpd1 also displayed antimicrobial activity against a panel of microorganisms. The pathway assay used in conjunction with assays for individual enzymes provides an efficient means of detecting and characterizing novel antimicrobial agents.
The emergence of antibiotic-resistant bacteria and newly described pathogens has created an urgent need for novel antibiotics. Because enzymes of the bacterial cell wall biosynthesis pathway do not have mammalian counterparts, they are valuable targets for new antimicrobial agents. The bacterial cell wall is comprised mainly of peptidoglycan, whose synthesis begins in the cytoplasm with the condensation of phosphoenolpyruvate (PEP) and UDP-N-acetylglucosamine (UDP-GlcNAc) to form UDP-GlcNAc-enolpyruvate, a reaction catalyzed by MurA. The sequential action of cytoplasmic enzymes leads to the formation of UDP-muramoyl pentapeptide, the substrate for MraY, a membrane-bound enzyme that catalyzes the formation of lipid I (7, 16). Subsequently, MurG and penicillin-binding proteins (PBPs) catalyze the formation of lipid II and cross-linked peptidoglycan, respectively (7, 16).
Many enzymes of the peptidoglycan pathway are essential, and disruption of their corresponding genes affects bacterial growth and viability (4, 8, 21). Gene products and intermediates of the pathway are the targets of several antibiotics in clinical use (9). For example, vancomycin, used against multidrug-resistant gram-positive bacteria, binds to the d-Ala-d-Ala moiety of the disaccharide pentapeptide substrate (10). The widely used β-lactams are irreversible inhibitors of PBPs (6). MurA is the target of naturally occurring small molecules and protein antibiotics (3). Fosfomycin, a broad-spectrum agent, irreversibly inactivates MurA in a time-dependent manner by covalently modifying Cys 115 (12, 15). Inactivation of MurA by fosfomycin in biochemical assays requires preincubation in the presence of UDP-GlcNAc (13, 15). Following formation of a binary complex with UDP-GlcNAc, MurA acquires an open conformation, which exposes its active site to fosfomycin binding (13, 15). Moreover, a capsid protein of RNA phage Qβ (A2) effects host lysis by inhibiting MurA (3). Inhibition of MraY and PBPs also causes bacterial lysis (2, 17). The binding of bactericidal antibiotics to their targets blocks cell growth but does not directly cause cell death (17); however, binding triggers signal transduction events leading to the deregulation of suicidal enzymes. Hence, the drug-induced bacterial lysis results from abnormal functioning of cellular autolytic enzymes (19).
Pathway assays have been described for the intracellular steps of the peptidoglycan pathway (MurA to MurF) utilizing purified enzymes (20, 21). Moreover, ether-treated bacteria and membrane assays have been used to test inhibitors of the late stages of the pathway, encompassing MraY, MurG, and PBPs (7, 10). However, a whole-cell assay for examining the pathway enzymes has not been described. We have developed a whole-cell peptidoglycan pathway assay that is sensitive to inhibitors of all the biosynthetic steps from UDP-GlcNAc to cross-linked peptidoglycan. With this assay, an inhibitor was identified and shown to block MurA catalytic activity.
MATERIALS AND METHODS
Bacterial strains, plasmid, and reagents.
Escherichia coli TOP10 was obtained from Invitrogen (San Diego, Calif.). Plasmid pGEX-6P-1, E. coli BL21, and uridine diphospho-N-acetyl-d-[UDP-14C]glucosamine (UDP-GlcNAc) were from Amersham Pharmacia Biotech (Piscataway, N.J.). Other bacterial strains were from the American Type Culture Collection (ATCC). Flavomycin was obtained from Hoechst (Frankfurt, Germany). All other antibiotics were from Sigma (St. Louis, Mo.). Bacitracin, flavomycin, and tunicamycin represented mixes of the same class of compounds, with variable molecular weight. Hence, the concentrations were expressed in micrograms per milliliter.
Cloning, expression and purification of murA.
The E. coli murA gene (14) was PCR amplified from E. coli ATCC 47076 (MG1655) chromosomal DNA with the following primers: 5′ CGGGATCCATGGATAAATTTCGTGTTCAGG 3′ (forward) and 5′ CCGCTCGAGTTATTCGCCTTTCACACGCTC 3′ (reverse). Following insertion of the murA gene in the BamHI and XhoI sites of pGEX-6P-1, the recombinant plasmid was transformed into E. coli TOP10 and subsequently into the expression strain, E. coli BL21. Chromosomal DNA and plasmid isolation, DNA desalting, and purification from agarose gels were performed with kits from Qiagen (Valencia, Calif.). Expression of recombinant MurA, purification of the protein, and removal of the glutathione S-transferase tag were performed by standard procedures described for an Amersham Pharmacia Biotech kit. The purification step was done with the glutathione S-transferase-glutathione affinity system and PreScission protease, following instructions from the manufacturer.
MurA assay.
UDP-GlcNAc-dependent release of inorganic phosphate from PEP was the measure of enzymatic activity (14). The initial concentrations of PEP and UDP-GlcNAc in the assay mixtures were 0.5 and 1 mM, respectively. Unless otherwise specified, enzyme (1 μM) and test compounds were preincubated for 15 min in the presence of UDP-GlcNAc prior to the addition of PEP.
Pathway assay utilizing whole cells.
E. coli ATCC 47076 cells subjected to freezing and thawing were utilized as a source of cell wall biosynthesis enzymes for the pathway assay. Cells were grown to mid-exponential phase in 3-liter Erlenmeyer flasks containing 300 ml of LB medium (10 g of Bacto-Peptone, 5 g of Bacto-yeast extract, and 10 g of NaCl per liter; pH adjusted to 7). The flasks were incubated at 200 rpm and 37°C. At an optical density (600 nm) of 0.5 to 1, the cells were harvested at 4°C (4,500 × g for 10 min) and suspended in ice-cold buffer containing 50 mM Tris (pH 7.5), 20 mM MgCl2, 1 mM β-mercaptoethanol, and 4% sorbitol. The volume was adjusted to yield a final optical density (600 nm) of 40, and aliquots were frozen slowly at −80°C and stored at that temperature until use. Prior to use, the cells were thawed on ice. In any instance, the cells were submitted to only one cycle of freezing and thawing. For wet-weight determinations, 100-μl aliquots were centrifuged at 10,000 × g for 5 min in preweighted Eppendorf tubes, the supernatant was removed, and the weight was determined for the cell pellet. Test compounds were preincubated for 15 min in 45 μl of a reaction mixture consisting of 0.2 mg of cells (wet weight), 2% dimethyl sulfoxide (DMSO), 80 mM Tris-Cl (pH 7.5), 16 mM MgCl2, 0.4 mM β-mercaptoethanol, and 4% sorbitol (mix 1). The reaction was started by the addition of 5 μl of 50 mM Tris-Cl (pH 7.5) containing randomly 14C-labeled UDP-GlcNAc. The production of peptidoglycan was also tested by using mix 1 plus 50 mM NH4Cl (10) and allowed to proceed within linear time ranges. After incubation at 32°C, the reaction was stopped with 50 μl of 8% sodium dodecyl sulfate, and the mixture was heated at 90°C for 25 min. The hot sodium dodecyl sulfate-insoluble material was filtered with 0.45-μm-pore-size surfactant-free mixed cellulose ester membranes (Millipore Corporation, Bedford, Mass.), and the radioactivity was measured with a TopCount NXT from Packard BioScience (Meriden, Conn.).
Drug susceptibility testing.
MICs were determined for a panel of microorganisms according to standard procedures (1). Briefly, bacterial cultures were inoculated in 96-well plates containing liquid medium with various concentrations of the test compounds. Growth was monitored by measuring the optical density of the culture after incubation at 37°C for 24 h.
RESULTS
Pathway assay utilizing whole cells.
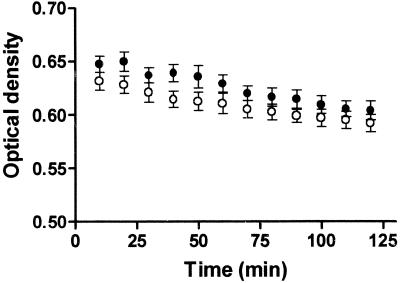
The enzymes involved in the committed steps of peptidoglycan biosynthesis can be tested simultaneously with an assay that utilizes radiolabeled UDP-GlcNAc as the substrate and whole E. coli cells as the source of enzymes. Peptidoglycan production by E. coli cells was tested with different buffers and various cell concentrations. Partial clogging of the filtration membrane resulted in increased background when a high cell concentration (0.4 mg [wet weight] of cells per reaction) was used. Incorporation of radioactivity into peptidoglycan was subsequently tested with 0.2 mg of cells and an incubation time of 25 min, which was within the linearity range for product formation. We next tested the effect of MgCl2 concentration on product formation and selected 10 mM as the concentration that allowed maximum product formation (data not shown). Nearly complete inhibition of the assay was observed at 70 mM MgCl2. Similar signals were observed when the assay was performed at pH 7, 7.5, and 8 (data not shown). Concentrations of [14C]UDP-GlcNAc ranging from approximately 0.05 to 0.25 μM were also tested in the assay. The 0.25 μM concentration (0.0125 μCi) was chosen for further work as the concentration approaching a plateau for product formation (data not shown). To test if cell lysis was responsible for the impaired product formation after longer incubation times, cells were incubated with the two different buffers, excluding the radiolabeled UDP-GlcNAc, and optical density (600 nm) was measured at intervals (Fig. 1). Only negligible decreases of the optical density measurements were observed during 2 h of incubation. Using this assay format, inhibition of peptidoglycan formation by several antibiotics was investigated (Fig. 2). Among the compounds tested, fosfomycin (disodium salt) was the strongest inhibitor, with a 50% inhibitory concentration (IC50) of 0.2 μg/ml. Inhibition by 50% or more was observed with 10-μg/ml concentrations of bacitracin, flavomycin, and vancomycin. The IC50s for d-cycloserine, penicillin G, and ampicillin were 1, 20, and 80 μg/ml, respectively (data not shown). Less than 25% inhibition was observed with tunicamycin, norfloxacin, kanamycin, and streptomycin at concentrations as high as 50 μM (data not shown).
FIG. 1.
Cell lysis as measured by optical density decrease (600 nm). Open circles, mix 1; closed circles, mix 1 supplemented with 50 mM NH4Cl. E. coli cells were subjected to one cycle of freezing and thawing.
FIG. 2.
Inhibition of the peptidoglycan pathway assay by fosfomycin (•), vancomycin (○), flavomycin (□), and bacitracin (▪).
Screening of compounds that inhibit peptidoglycan biosynthesis.
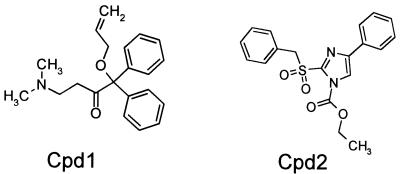
Inhibition of the peptidoglycan assay was tested with a sublibrary of 600 compounds. Two structures that inhibited peptidoglycan formation were identified (Fig. 3). Cpd1 and Cpd2 at 50 μM inhibited the assay by 25 and 50%, respectively.
FIG. 3.
Chemical structures of peptidoglycan biosynthesis inhibitors screened with a pathway assay utilizing whole E. coli cells as a source of enzymes.
MurA inhibition by Cpd1.
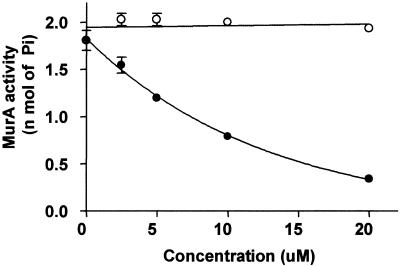
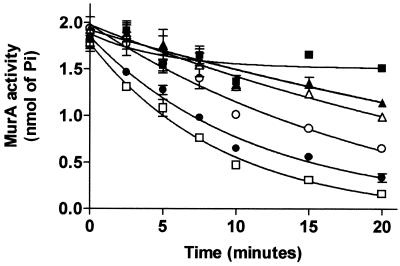
In an attempt to identify the target of Cpd1 and Cpd2, both compounds were tested with a MurA activity assay that measures the release of inorganic phosphate, and only Cpd1 inhibited the reaction (IC50 = 6 μM). The inhibition of MurA by Cpd1 is dependent on preincubation in the presence of UDP-GlcNAc (Fig. 4). Figure 5 shows the time-dependent inhibition of MurA by Cpd1. Cpd1 at concentrations from 2.5 to 30 μM was preincubated with the reaction mixtures for various intervals prior to the addition of substrate.
FIG. 4.
MurA inactivation by Cpd1 following preincubation in the presence (closed circles) or absence (open circles) of 1 mM UDP-GlcNAc.
FIG. 5.
Time-dependent inactivation of MurA during preincubation with Cpd1 at 2.5 (▴), 5 (▵), 10 (○), 20 (•), or 30 (□) μM or with DMSO (▪). The compound was dissolved in 100% DMSO.
Antimicrobial activity of Cpd1 and Cpd2.
Antimicrobial activity was tested against a panel of microorganisms. Cpd2 presented no antimicrobial activity at 12.2 μg/ml. Cpd1 inhibited growth of several microorganisms, although at 32.2 μg/ml no growth inhibition of E. coli was observed (Table 1).
TABLE 1.
Susceptibility of a panel of microorganisms to MurA inhibitors
| Microorganism | MIC (μg/ml)
|
|
|---|---|---|
| Cpd2 | Cpd1 | |
| Escherichia coli ATCC 25922 | >12.2 | >32.3 |
| Haemophilus influenzae ATCC 49766 | >12.2 | 32.3 |
| Moraxella catarrhalis ATCC 25238 | >12.2 | 8.1 |
| Staphylococcus aureus ATCC 13709 | >12.2 | 16.1 |
| Streptococcus pneumoniae ATCC 49619 | >12.2 | 16.1 |
| Candida albicans ATCC 90028 | >12.2 | 32.3 |
DISCUSSION
The peptidoglycan pathway assay allows enzymes of the essential steps of bacterial cell wall biosynthesis to be screened simultaneously. Peptidoglycan production increases with the concentration of the exogenous substrate, approaching a plateau at approximately 0.25 μM [14C]UDP-GlcNAc. This may be due to feedback inhibition of MurA by intermediates of the pathway (22). Feedback inhibition of MurA at higher substrate concentrations would be consistent with differential catalytic activities of MurA and MurG (or higher intracellular levels of MurA) leading to accumulation of intermediates upstream of lipid II.
Several antibiotics that target enzymes involved in peptidoglycan synthesis inhibited the assay utilizing E. coli cells. Among the antibiotics tested, fosfomycin presented the lowest IC50. It is a broad-spectrum antibiotic clinically used to treat urinary tract infections, and it inactivates the first enzyme of the pathway, MurA (9, 15). The inhibition of the assay by fosfomycin indicated that the reaction in the freeze-thawed cells begins with the condensation of 14C-labeled UDP-GlcNAc and PEP catalyzed by MurA. Perhaps the internal pool of UDP-muramoyl pentapeptide was not sufficient to allow formation of lipid I, catalyzed by MraY (5, 7, 16). Alternatively, different catalytic efficiencies of MurA and MurG by the substrate UDP-GlcNAc may explain the preferential utilization of the radiolabeled substrate by the first enzyme of the pathway. The freeze-thawing process may induce some alteration of the E. coli permeability barrier (18), allowing limited inhibition of the assay by vancomycin, which binds d-Ala-d-Ala and exerts antimicrobial activity mainly against gram-positive organisms. This interpretation is consistent with the strong inhibition observed with fosfomycin, an antibiotic potent against E. coli. Fosfomycin completely inhibited peptidoglycan formation, suggesting that radiolabeled UDP-GlcNAc was channeled into the cell wall biosynthesis pathway exclusively. Antibiotics that inhibit protein synthesis (kanamycin and streptomycin) or DNA replication (norfloxacin) had no effect on the assay. Bacitracin prevents dephosphorylation of undecaprenyl pyrophosphate, while flavomycin (an antibiotic of the moenomycin family) inhibits transglycosylation (9, 11). Both antibiotics have been shown to inhibit peptidoglycan formation by ether-treated bacteria (11) at concentrations lower than the inhibitory concentrations observed with the whole-cell assay. The E. coli permeability barrier was likely compromised by ether treatment, making the enzyme (PBP) and substrate (undecaprenyl pyrophosphate) more accessible to the inhibitors.
Both Cpd1 and Cpd2 inhibited the peptidoglycan pathway assay. Subsequent tests indicated that MurA is a target of Cpd1. Cp2 did not inhibit MurA and presumably inactivates another enzyme in the peptidoglycan biosynthesis pathway. Moreover, kinetic studies provided evidence that Cpd1 is a time-dependent inhibitor of MurA. The possibility that Cpd1 can also inactivate bacterial enzymes besides MurA cannot be ruled out. However, the pattern of MurA inhibition by Cpd1 is similar to the one observed with fosfomycin (14, 15). Upon binary complex formation with UDP-GlcNAc, MurA adopts an open conformation, exposing the active site and allowing covalent binding of fosfomycin (13). Inhibition of MurA activity by Cpd1 also requires preincubation in the presence of UDP-GlcNAc.
Cpd1 did not inhibit E. coli growth at the highest concentration tested (32 μg/ml) and was a poor inhibitor of the pathway assay. Taken together, these observations suggest that the E. coli permeability barrier (18) is to some extent still functional in the freeze-thawed cells, limiting inhibition of MurA by Cpd1 in the pathway assay. Hence, compounds more potent in the pathway assay are more likely to represent broad-spectrum antibiotics.
Acknowledgments
We Thank H. Liu for cloning and purifying recombinant MurA. We also thank K. Amsler and L. Foster for MIC determinations and B. Schwartz for critical reading of the manuscript.
REFERENCES
- 1.Amsterdan, D. 1966. Susceptibility testing of antimicrobials in liquid medium, p. 52-111. In V. Lorian (ed.), Antibiotics in laboratory medicine, 4th ed. Williams & Wilkins, Baltimore, Md.
- 2.Bernhardt, T. G., D. K. Struck, and R. Young. 2001. The lysis protein E of φX174 is a specific inhibitor of the MraY-catalyzed step in peptidoglycan synthesis. J. Biol. Chem. 276:6093-6097. [DOI] [PubMed] [Google Scholar]
- 3.Bernhardt, T. G., I.-N. Wang, D. K. Struck, and R. Young. 2001. A protein antibiotic in the phage Qβ virion: diversity in lysis targets. Science 292:2326-2329. [DOI] [PubMed] [Google Scholar]
- 4.Boyle, D. S., and W. D. Donachie. 1998. MraY is an essential gene for cell growth in Escherichia coli. J. Bacteriol. 180:6429-6432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Branstrom, A. A., S. Midha, C. B. Longley, K. Han, E. R. Baizman, and H. R. Axelrod. 2000. Assay for identification of inhibitors for bacterial MraY translocase and MurG transferase. Anal. Biochem. 280:315-319. [DOI] [PubMed] [Google Scholar]
- 6.Bulychev, A., I. Massova, K. Miyashita, and S. Mobashery. 1997. Nuances of mechanisms and their implications for evolution of the versatile β-lactamase activity: from biosynthetic enzymes to drug resistance factors. J. Am. Chem. Soc. 119:7619-7625. [Google Scholar]
- 7.Chandrakala, B., B. C. Elias, U. Mehra, N. S. Umapathy, P. Dwarakanath, T. S. Balganesh, and S. M. Desousa. 2001. Novel scintillation proximity assay for measuring membrane-associated steps of peptidoglycan biosynthesis in Escherichia coli. Antimicrob. Agents Chemother. 45:768-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denome, S. A., P. K. Elf, T. A. Henderson, D. E. Nelson, and K. D. Young. 1999. Escherichia coli mutants lacking all possible combinations of eight penicillin binding proteins: viability, characteristics, and implications for peptidoglycan synthesis. J. Bacteriol. 181:3981-3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gadebush, H. H., E. O. Stapley, and S. B. Zimmerman. 1992. The discovery of cell wall active antibacterial antibiotics. Crit. Rev. Biotechnol. 12:225-243. [DOI] [PubMed] [Google Scholar]
- 10.Ge, M., Z. Chen, H. R. Onishi, J. Kohler, L. L. Silver, R. Kerns, S. Fukuzawa, C. Thompson, and D. Kahne. 1999. Vancomycin derivatives that inhibit peptidoglycan biosynthesis without binding d-Ala-d-Ala. Science 284:507-511. [DOI] [PubMed] [Google Scholar]
- 11.Goldman, R. C., and D. Range. 2000. Inhibition of transglycosylation involved in bacterial peptidoglycan synthesis. Curr. Med. Chem. 7:801-820. [DOI] [PubMed] [Google Scholar]
- 12.Kim, D. H., W. J. Lees, K. E. Kempsell, W. S. Lane, K. Duncan, and C. T. Walsh. 1996. Characterization of a Cys115 to Asp substitution in the Escherichia coli cell wall biosynthetic enzyme UDP-GlcNAc enolpyruvyl transferase (MurA) that confers resistance to inactivation by the antibiotic fosfomycin. Biochemistry 35:4923-4928. [DOI] [PubMed] [Google Scholar]
- 13.Krekel, F., C. Oecking, N. Amrhein, and P. Macheroux. 1999. Substrate and inhibitor-induced conformational changes in the structurally related enzymes UDP-N-acetylglucosamine enolpyruvyl transferase (MurA) and 5-enolpyruvylshikimate 3-phosphate synthase (EPSPS). Biochemistry 38:8864-8878. [DOI] [PubMed] [Google Scholar]
- 14.Marquardt, J. L., D. A. Siegele, R. Kolter, and C. T. Walsh. 1992. Cloning and sequencing of Escherichia coli murZ and purification of its product, a UDP-N-acetylglucosamine enolpyruvyl transferase. J. Bacteriol. 174:5748-5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marquardt, J. L., E. D. Brown, W. S. Lane, T. M. Haley, Y. Ichikawa, C.-H. Wong, and C. T. Walsh. 1994. Kinetics, stoichiometry, and identification of the reactive thiolate in the inactivation of UDP-GlcNAc enolpyruvoyl transferase by the antibiotic fosfomycin. Biochemistry 33:10646-10651. [DOI] [PubMed] [Google Scholar]
- 16.Navarre, W. W., and O. Schneewind. 1999. Surface proteins of gram-positive bacteria and mechanisms of their targeting to the cell wall envelope. Microbiol. Mol. Biol. Rev. 63:174-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Novak, R., E. Charpentier, J. S. Braun, and E. Tuomanen. 2000. Signal transduction by a death signal peptide: uncovering the mechanism of bacterial killing by penicillin. Mol. Cell 5:49-57. [DOI] [PubMed] [Google Scholar]
- 18.Sulavick, M. C., C. Houseweart, C. Cramer, N. Jiwani, N. Murgolo, J. Greene, B. DiDomenico, K. J. Shaw, G. H. Miller, R. Hare, and G. Shimer. 2001. Antibiotic susceptibility profiles of Escherichia coli strains lacking multidrug efflux pump genes. Antimicrob. Agents Chemother. 45:1126-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomasz, A., A. Albino, and E. Zanati. 1970. Multiple antibiotic resistance in a bacterium with suppressed autolytic system. Nature 227:138-140. [DOI] [PubMed] [Google Scholar]
- 20.Trias, J., and Y. Zhengyu. 1999. Mining bacterial cell wall biosynthesis with new tools: multitarget screens. Drug Res. Updates 2:358-362. [DOI] [PubMed] [Google Scholar]
- 21.Wong, K. K., D. W. Kuo, R. M. Chabin, C. Fournier, L. D. Gregnas, S. T. Waddell, F. Marsilio, B. Leiting, and D. L. Pompliano. 1998. Engineering a cell-free murein biosynthetic pathway: combinatorial enzymology in drug discovery. J. Am. Chem. Soc. 120:13527-13528. [Google Scholar]
- 22.Zemell, R. I., and R. A. Anwar. 1975. Pyruvate-uridine diphospho-N-acetylglucosamine transferase. Purification to homogeneity and feedback inhibition. J. Biol. Chem. 250:3185-3192. [PubMed] [Google Scholar]