Abstract
The nucleotide phosphonates cidofovir (CDV) and cyclic cidofovir (cCDV) are potent antiviral compounds when administered parenterally but are not well absorbed orally. These compounds have been reported to have activity against orthopoxvirus replication in vitro and in animal models when administered parenterally or by aerosol. To obtain better oral activity, we synthesized a novel series of analogs of CDV and cCDV by esterification with two long-chain alkoxyalkanols, 3-hexadecyloxy-1-propanol (HDP-CDV; HDP-cCDV) or 3-octadecyloxy-1-ethanol (ODE-CDV; ODE-cCDV). Their activities were evaluated and compared with those of CDV and cCDV in human foreskin fibroblast (HFF) cells infected with vaccinia virus (VV) or cowpox virus (CV) using a plaque reduction assay. The 50% effective concentrations (EC50s) against VV in HFF cells for CDV and cCDV were 46.2 and 50.6 μM compared with 0.84 and 3.8 μM for HDP-CDV and HDP-cCDV, respectively. The EC50s for ODE-CDV and ODE-cCDV were 0.20 and 1.1 μM, respectively. The HDP analogs were 57- and 13-fold more active than the parent nucleotides, whereas the ODE analogs were 231- and 46-fold more active than the unmodified CDV and cCDV. Similar results were obtained using CV. Cytotoxicity studies indicated that although the analogs were more toxic than the parent nucleotides, the selective index was increased by 4- to 13-fold. These results indicate that the alkoxyalkyl esters of CDV and cCDV have enhanced activity in vitro and need to be evaluated for their oral absorption and efficacy in animal models.
Since smallpox was considered to be eradicated in the 1970s, there has been little activity in developing antiviral agents for this infection (10). However, in view of the threat of bioterrorism using variola virus or other orthopoxviruses, such as monkeypox virus, which continues to infect humans in central Africa, there is a renewed need to develop antiviral agents for these viruses (3, 11, 12, 17, 18, 24). For many years the laboratory of Erik De Clercq and other laboratories have utilized in vitro and animal models with vaccinia virus (VV) to screen potential antiviral compounds for activity against poxviruses and have identified a few active agents. Methisazone, ribavirin, idoxuridine, interferon, interferon inducers, S2442, and cidofovir (CDV) have been identified as potential therapies for these infections (7, 8, 9, 20, 21, 23). Of particular interest was the finding that CDV and other phosphonate nucleotides were inhibitory to this group of viruses, including VV, cowpox virus (CV), camelpox virus, monkeypox virus, and variola virus (J. W. Huggins, personal communication). The activity of CDV is of particular interest as a potential therapy for smallpox, as it is already approved for the treatment of cytomegalovirus (CMV) infections and has been shown to have activity in animal models using VV and CV (2, 22, 26, 27). We have confirmed the activity of CDV against both VV and CV in tissue culture and animal models in our laboratory (4) and report here the results of some new alkoxyalkyl esters of CDV and cyclic CDV (cCDV). Although CDV and cCDV are potent inhibitors of orthopoxvirus replication in vitro and are highly effective in animal models when inoculated parenterally, they are absorbed poorly when given orally (1, 5).
Previous studies from our group have shown that alkylglycerol phosphate or alkoxypropyl phosphate esters of acyclovir (14) and ganciclovir (16) have greater oral bioavailability than the parent compounds in rodents. Furthermore, these compounds are active orally in animal models of herpesvirus disease (16) and hepatitis (15). To obtain better oral activity with CDV, we synthesized a new series of analogs by esterification using two long-chain alkoxyalkanols, 3-hexadecyloxy-1-propanol (HDP-CDV; HDP-cCDV) and 3-octadecyloxy-1-ethanol (ODE-CDV; ODE-cCDV) (Fig. 1). We then compared their activities with those of CDV and cCDV in human foreskin fibroblast (HFF) cells infected with strains of VV or CV using a plaque reduction assay. The cytotoxicities of the compounds were determined using a neutral-red uptake (50% cytotoxic concentration [CC50]) or cell proliferation (50% inhibitory concentration [IC50]) assay.
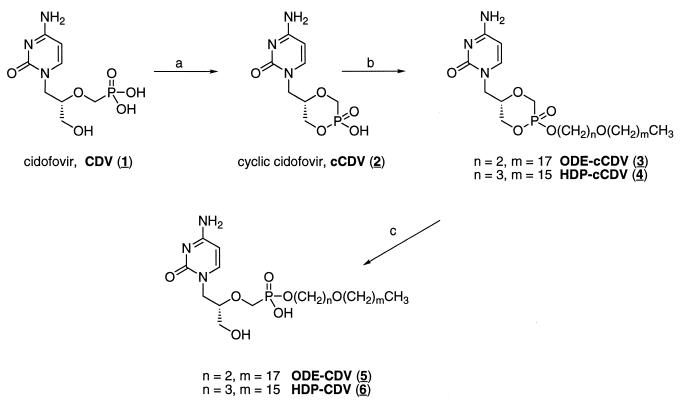
FIG. 1.
Synthesis and structures of alkoxyalkyl analogs of CDV and cCDV. The arrows indicate the following reagents: (a) N,N-dicyclohexyl-morpholinocarboxamide, N,N-dicyclohexylcarbodiimide, pyridine, 100°C; (b) 1-bromo-3-octadecyloxyethane (ODE), or 1-bromo-3-hexadecyloxypropane (HDP), N,N-dimethylformamide, 80°C; (c) 0.5 M NaOH. The numbers in parentheses are the compound numbers (see the text).
MATERIALS AND METHODS
Chemistry. (i) General.
Thin-layer chromatography was performed on Analtech 250-μm-thick silica gel GF Uniplates and visualized by UV, phospray (Supelco, Bellafonte, Pa.), and charring. Chromatographic purification was done by the flash method using Merck silica gel 60, 240 to 400 mesh. 1H and 31P nuclear magnetic resonance (NMR) spectra were recorded at 400 MHz on a Varian HG-400 spectrophotometer with tetramethylsilane (internal) or 85% D3PO4 in D2O (external) as 1H and 31P references (0.00 ppm), respectively. Mass spectroscopy (MS) was performed by Mass Consortium (San Diego, Calif.). CDV (Vistide) (compound 1) was purchased from a retail pharmacy or was provided by Gilead Sciences, Inc. (Foster City, Calif.).
(ii) cCDV dicyclohexyl-morpholinocarboxamidine salt (compound 2).
cCDV was prepared from CDV as described previously (1) except that the compound was isolated as the dicyclohexyl-morpholinocarboxamidine salt. Thus, to a strirred suspension of CDV (1.0 g; 3.17 mmol) in N,N-dimethylformamide (25 ml) was added N,N-dicyclohexyl-4-morpholinecarboxamidine (1.03 g; 3.5 mmol). The reaction mixture was stirred for 12 h at room temperature, during which the CDV dissolved. This solution was then added slowly to a hot pyridine (25 ml) solution of 1,3-dicyclohexyl carbodiimide (1.63 g; 7.9 mmol). The mixture was stirred at 100°C for 16 h, cooled to room temperature, and filtered, and the filtrate was concentrated under vacuum. The residue was purified by chromatography. Elution with 60% MeOH-40% CH2Cl2 gave compound 2 as a white solid.
(iii) 1-Bromo-3-octadecyloxyethane.
To a cooled (0°C) solution of 3-octadecyloxy-1-ethanol (10 g; 31.8 mmol) and carbon tetrabromide (21.1 g; 64 mmol) was added triphenylphosphine (21.3 g; 81 mmol) in 4-g portions over 30 min. The reaction mixture was stirred for 45 min at 0°C and then for 1 h at room temperature. The reaction mixture was concentrated, and the residue was dissolved in ether. After being stirred for 1 h, the mixture was filtered, and the filtrate was concentrated. The residue was purified by flash chromatography. Elution with 90% hexane-10% ethyl acetate yielded 8.0 g (67%). 1H NMR 0.88 (t, 3H), 1.25 (br s, 32H), 1.57 (m, 2H), 3.47 (q, 2H), 3.74 (t, 2H).
(iv) 1-Bromo-3-hexadecyloxypropane.
To a cooled (0°C) solution of 3-hexadecyloxy-1-propanol (4.7g; 16 mmol) and carbon tetrabromide (11.2 g; 34 mmol) was added triphenylphosphine in 2-g portions over 30 min. The reaction was stirred for 45 min at 0°C and then for 1 h at room temperature. The brominated product was isolated as described above. 1H NMR 0.88 (t, 3H), 1.25 (broad s, 28H), 1.56 (m, 2H), 2.09 (p, 2H), 3.43 (t, 2H), 3.52 (q, 2H).
(v) cCDV-octadecyloxyethyl ester (compound 3).
A mixture of compound 2 (1.0 g; 1.8 mmol) and 1-bromo-3-octadecyloxyethane (3.0 g; 7.9 mmol) in N,N-dimethylformamide (35 ml) was stirred and heated to 80°C for 6 h. The mixture was concentrated, and the residue was purified by flash chromatography. Elution with 10% MeOH-90% CH2Cl2 yielded 320 mg (33%). 1H NMR (dimethyl sulfoxide [DMSO]-d6) 0.87 (t, 3H), 1.23 (broad s, 32H), 1.47 (m, 2H), 3.55 B 4.20 (m, 11H), 5.65 (dd, 1H), 7.18 and 7.04 (broad s, 1 H), 7.55 and 7.45 (d, 1H), 8.30 (broad s, 2H); 31P NMR +13.88 and +12.62; MS electrospray ionization [EI]) m/z 558 (M + H)+, 556 (M − H)−.
(vi) cCDV-hexadecyloxypropyl ester (compound 4).
A mixture of compound 2 (500 mg; 0.9 mmol) and 1-bromo-3-hexadecyloxypropane (1.8 g; 5 mmol) in N,N-dimethylformamide (35 ml) was stirred and heated (80°C) for 6 h. The mixture was then concentrated in vacuo, and the residue was purified by flash chromatography. Elution with 10% MeOH-CH2Cl2 afforded 150 mg of a white powder (31% yield). High-performance liquid chromatography, thin-layer chromatography, and spectroscopic analysis showed the presence of two diastereomeric (axial and equatorial) alkylation products. 1H NMR (DMSO-d6) 0.85 (t, 3H), 1.23 (broad s, 28H), 1.47 (m, 2H), 1.84 (p, 2H), 3.55 B 4.20 (m, 11H), 5.65 (dd, 1H), 7.18 and 7.04 (broad s, 1 H), 7.55 and 7.45 (d, 1H), 8.30 (broad s, 2H); 31P NMR +13.88 and +12.62; MS (EI) m/z 544 (M + H)+, 542 (M − H)−.
(vii) CDV-octadecyloxyethyl ester (compound 5).
Compound 3 (160 mg; 0.3 mmol) was dissolved in 0.5 M NaOH (20 ml) and stirred at room temperature for 1.5 h. The solution was neutralized with acetic acid. The precipitate was collected by filtration. 31P NMR +13.98; MS (EI) m/z 598 (M + Na)+, 574 (M − H)−.
(viii) CDV-hexadecyloxypropyl ester (compound 6).
Compound 4 (130 mg; 0.23 mmol) was dissolved in 0.5 M NaOH (5 ml) and stirred at room temperature for 1.5 h. The solution was neutralized with acetic acid, and the precipitate was isolated by filtration and then purified by flash column chromatography. The product (105 mg) was eluted with 70:30 CH2Cl2-MeOH. 1H NMR (DMSO-d6) 0.86 (t, 3H), 1.24 (broad s, 28H), 1.47 (m, 2H), 1.73 (p, 2H), 3.20 B 3.89 (m, 11H), 5.72 (m, 1H), 7.21 (d, 1 H), 7.54 (d, 1H), 8.23 (broad s, 2H); 31P NMR +13.98; MS (EI) m/z 584 (M + Na)+, 560 (M − H)−.
Virus pool preparation.
The VV strain Copenhagen and CV strain Brighton stock pools were obtained from John Huggins of the U.S. Army Medical Research Institute for Infectious Diseases, Frederick, Md. These pools had been prepared in Vero cells and were diluted 1:50 in our laboratory to provide working stocks. VV strains WR, Elstree, IHD, and NYC were obtained from the American Type Culture Collection and propagated in HFF cells.
Plaque reduction assay for efficacy.
Two days prior to use, HFF cells were plated on six-well plates and incubated at 37°C with 10% CO2 and 90% humidity. On the day of assay, the drugs were made up at twice the desired concentration in 2× minimal essential medium (MEM) containing 5% fetal bovine serum (FBS) and antibiotics and diluted serially 1:5 in 2× MEM to provide six concentrations of drug. The initial starting concentration was usually 200 μM and ranged down to 0.06 μM. The virus to be used was diluted in MEM containing 10% FBS to a desired concentration which would give 20 to 30 plaques per well. The medium was then aspirated from the wells, and 0.2 ml of virus was added to each well in triplicate, with 0.2 ml of medium being added to drug toxicity wells. The plates were incubated for 1 h with shaking every 15 min. After the incubation period, an equal amount of 1% agarose was added to an equal volume of each drug dilution. This gave final drug concentrations beginning with 100 μM and ending with 0.03 μM and a final agarose overlay concentration of 0.5%. The drug-agarose mixture was added to each well in 2-ml volumes, and the plates were incubated for 3 days, after which the cells were stained with a 1.5% solution of neutral red. After a 5- to 6-h incubation period, the stain was aspirated and the plaques were counted using a stereomicroscope at ×10 magnification. The MacSynergy II, version 1, computer program was used to calculate the 50% effective concentration (EC50).
Neutral-red uptake assay for toxicity.
Twenty-four hours prior to the assay, HFF cells were plated on 96-well plates at a concentration of 2.5 × 105 per ml. After 24 h, the medium was aspirated and 125 μl of drug was added to the first row of wells and then diluted serially 1:5 using the Beckman BioMek liquid-handling system. After the addition of the drug, the plates were incubated for 7 days in a CO2 incubator at 37°C. At that time, the medium with drug was aspirated, and 200 μl of 0.01% neutral red in phosphate-buffered saline (PBS)/well was added and incubated for 1 h. The dye was aspirated, and the cells were washed with PBS using a Nunc plate washer. After the PBS was removed, 200 μl of 50% ethanol-1% glacial acetic acid (in H2O)/well was added. The plates were placed on a rotary shaker for 15 min, and the optical densities were read at 540 nm on a Bio-tek plate reader. The CC50 was calculated using the program described previously.
Cell proliferation assay for toxicity.
Twenty-four hours prior to the assay, HFF cells were seeded in 6-well plates at a concentration of 2.5 × 104 per well in MEM containing 10% FBS. On the day of the assay, the drugs were diluted serially in MEM containing 10% FBS at increments of 1:5 covering a range from 100 to 0.03 μM. The medium from the wells was aspirated, and 2 ml of each drug concentration was then added to each well. The cells were incubated in a CO2 incubator at 37°C for 72 h. The medium-drug solution was removed, and 1 ml of 0.25% trypsin was added to each well and incubated until the cells started to come off the plate. The cell-trypsin mixture was pipetted up and down vigorously to provide a homogenous cell suspension, 0.2 ml of the mixture was added to 9.8 ml of Isoton III, and the cells were counted using a Coulter Counter. Each sample was counted three times with two replicate wells per sample. The IC50 was also calculated as described above.
RESULTS
The potential for use of a poxvirus as a bioterrorism agent or for the accidental spread of an indigenous pathogen, such as monkeypox virus, has resulted in a renewed effort in the development of both new antiviral therapies and new vaccines for prophylactic or therapeutic use. As part of an initial screening effort to identify an antiviral agent that could be rapidly developed for the treatment of poxvirus infections, we evaluated the activities of most of the currently approved antiviral drugs against both VV and CV infections in vitro. Since our laboratory uses HFF cells in our antiviral screening assays and most prior work has been carried out in Vero cells, we first compared the activities of a number of nucleosides and nonnucleosides that are approved for treatment of other viral infections in both HFF and Vero cells. The activities of these compounds against VV and CV are summarized in Table 1. The only compounds that exhibited significant activity in these assays were CDV, cCDV, fialuridine, and idoxuridine. In addition, the protease inhibitors nevirapine, nelfinavir, indinavir, saquinavir, and ritonavir were evaluated and had no activity. Since fialuridine and idoxuridine are both considered to be too toxic for parenteral administration to humans, CDV, which is currently approved for use in CMV infections in the immunocompromised host, appears to be the best choice for development for use against a potential poxvirus outbreak. Although CDV also has significant side effects, it may be acceptable for short-term use.
TABLE 1.
Efficacies of antiviral agents against VV and CV in HFF and Vero cells
| Drug | EC50 (μM)
|
|||
|---|---|---|---|---|
| VV
|
CV
|
|||
| HFF | Vero | HFF | Vero | |
| Acyclovir | >144 | >144 | >444 | >444 |
| Ganciclovir | >392 | NTb | NT | NT |
| CDV | 23 ± 4.1a | 30 ± 12.6a | 48 ± 1.8a | 45 ± 7.9a |
| cCDV | 21 ± 4.9a | 90 ± 46a | 51 ± 4.2a | 132 ± 9.6a |
| Ribavirin | 281 | NT | >410 | >246 |
| Fialuridine | 1.5 ± 0.05a | NT | 0.24 ± 0.08a | NT |
| Zidovudine | >374 | >374 | >374 | >374 |
| Lamivudine | >436 | >436 | >436 | >436 |
| Didanosine | >423 | >423 | >423 | >423 |
| Zalcitibine | >474 | >474 | >474 | >474 |
| Stavudine | >446 | >446 | >446 | >446 |
| Idoxuridine | 6.0 ± 0.2a | NT | 2.0 ± 0.20a | NT |
Values are the means of two assays ± standard deviations.
NT, not tested.
Based on our results with CDV, as well as those in the literature (9), we next evaluated other phosphonate nucleotides that are under evaluation for other diseases, such as AIDS and hepatitis. The results of these evaluations indicated that CDV, cCDV, adefovir dipivoxil, and 3-hydroxy-2-phosphonylmethoxy propyl adenine all had activity against VV and CV (data not presented). In essentially all cases, the compounds were as active, if not more so, in HFF cells as in Vero cells, so all subsequent assays were performed using HFF cells.
In the next experiment, we compared the activities of the two parent nucleotides with the newly synthesized alkoxyalkyl analogs, and the results are presented in Table 2. Both CDV and cCDV required about 40 to 50 μM to inhibit the replication of either VV or CV by 50%. In contrast, the alkoxyalkyl analogs, HDP-CDV and HDP-cCDV, were active at about 0.8 to 4.0 μM, and the ODE-CDV and ODE-cCDV analogs were active at 0.2 to 1.0 μM, respectively. The new analogs of CDV were 58- to 231-fold more active than the unmodified CDV against VV and 75- to 150-fold more active than CDV against CV. The alkoxyalkyl adducts of cCDV were about 13- to 97-fold more active against these viruses than the parent cCDV.
TABLE 2.
Activities of phosphonate nucleotides and alkoxyalkyl analogs against VV and CV in HFF cells
| Compound | EC50 (μM)a
|
|
|---|---|---|
| VV Copenhagen | CV Brighton | |
| CDV | 46.2 ± 11.9 | 44.7 ± 6.3 |
| HDP-CDV | 0.8 ± 0.4 | 0.6 ± 0.3 |
| ODE-CDV | 0.2 ± 0.1 | 0.3 ± 0.3 |
| cCDV | 50.6 ± 13.1 | 48.3 ± 8.0 |
| HDP-cCDV | 3.8 ± 1.5 | 2.1 ± 1.9 |
| ODE-cCDV | 1.1 ± 1.0 | 0.5 ± 0.1 |
Values are the means of two assays ± standard deviations.
To guard against the possibility that the Copenhagen strain is not representative of VV strains, we determined the activities of the six phosphonate compounds against five strains of VV, and the results are summarized in Table 3. All six of the nucleotides were similarly active against the five strains of VV; however, the IHD and NYC strains appeared to be more susceptible than the Copenhagen, WR, or Elstree strain. Since there is only a single strain of CV, similar comparisons could not be made. The EC50s for HDP-CDV ranged from 0.20 to 1.2 μM, while ODE-CDV EC50s were 0.10 to 0.40 μM, representing 28- to 209-fold increases in activity versus CDV for these same strains. The HDP- and ODE-cCDV analogs were generally slightly less active, with EC50s ranging from 0.10 to 5.6 and 0.03 to 1.1 μM, respectively, which represent increases of 11- to 343-fold over the activity of cCDV.
TABLE 3.
Activities of phosphonate nucleotides and alkoxyalkyl analogs against strains of VV
| Compound | EC50 (μM)a
|
||||
|---|---|---|---|---|---|
| Copenhagen | WR | Elstree | IHD | NYC | |
| CDV | 46.2 ± 11.9 | 45.8 ± 16.6 | 41.6 ± 22.4 | 13.4 ± 5.6 | 10.1 ± 1.3 |
| HDP-CDV | 0.8 ± 0.4 | 1.1 ± 1.0 | 1.2 ± 0.8 | 0.2 ± 0.0 | 0.4 ± 0.0 |
| ODE-CDV | 0.2 ± 0.1 | 0.24 ± 0.2 | 0.4 ± 0.1 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| cCDV | 50.6 ± 13.1 | 58.8 ± 10.9 | 41.6 ± 19.5 | 10.9 ± 1.3 | 10.3 ± 1.3 |
| HDP-cCDV | 3.8 ± 1.5 | 5.6 ± 1.3 | 3.8 ± 2.7 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| ODE-cCDV | 1.1 ± 1.0 | 0.58 ± 0.2 | 0.5 ± 0.4 | 0.04 ± 0.02 | 0.03 ± 0 |
Values are the means of two assays ± standard deviations.
Since the evaluation of the toxicity of a new compound is an integral part of any drug development scheme, we next determined the cellular cytotoxicities of these compounds and their propensities for inhibition of cell proliferation. The cellular cytotoxicity (CC50) and cell proliferation (IC50) values for these compounds are listed in Table 4. In these assays, CDV and cCDV had relatively similar toxicities; however, the HDP analogs were about three- to ninefold more toxic than CDV or cCDV. The ODE analogs were 4- to 19-fold more toxic than either CDV or cCDV. The activity of a new compound, taking into account both its efficacy and toxicity, is often expressed as a selective index (SI), that is, the CC50 divided by the EC50. The SI values for the six phosphonate nucleotide compounds are summarized in Table 5. The SI values for both CDV and cCDV were about 6, whereas those for the HDP analogs of CDV and cCDV were about 30. The SI values for the ODE analogs were 40 to 65, indicating that the analogs, although more toxic than the parent compounds, were more efficacious, which resulted in higher SI values.
TABLE 4.
Cellular cytotoxicities (CC50) and inhibition of cell proliferation (IC50) of phosphonate nucleotides and alkoxyalkyl analogs in HFF cells
| Compound | CC50 (μM)a (neutral-red uptake) | IC50 (μM)a (cell proliferation) |
|---|---|---|
| CDV | 278.4 ± 9.2 | 19.5 ± 8.7 |
| HDP-CDV | 31 ± 2.1 | 1.7 ± 1.8 |
| ODE-CDV | 14.3 ± 9.7 | 0.5 ± 0.3 |
| cCDV | >302 ± 0 | 27.8 |
| HDP-cCDV | >100 ± 0 | 11.7 ± 4.5 |
| ODE-cCDV | 47.8 ± 57.3 | 2.2 ± 0.5 |
Values are the means of two assays ± standard deviations.
TABLE 5.
SI values of phosphonate nucleotides and alkoxyalkyl analogs in HFF cells
DISCUSSION
There is at present no approved antiviral therapy that could be rapidly deployed in the event of a bioterrorism attack using a poxvirus or the unexpected spread of an indigenous agent like monkeypox virus to other parts of the world. Initial efforts in drug development for poxvirus infections have focused on the identification of compounds that are already approved for use against some other disease. Historically, a few compounds, including methisazone, ribavirin, idoxuridine, and fialuridine, have been reported to have various degrees of activity against a surrogate virus, VV (9). Our results also confirmed the activities of idoxuridine and fialuridine against both VV and CV; however, ribavirin was only slightly active in our assays. For a variety of reasons, including lack of clear efficacy, toxicity, or availability, none of these compounds are good possibilities for development for use against poxvirus infections.
Investigators from the laboratory of Erik DeClercq (8, 9, 20) first reported that a group of compounds often referred to as phosphonate nucleotides had activity against VV. These results have been confirmed by other laboratories, and the activity has been extended to other poxviruses, including cowpox virus (2, 4, 26), camel pox virus, monkey pox virus, and variola virus, the causative agent of smallpox (Huggins, personal communication). The best-known member of this class of compounds is CDV, which is currently approved for treatment of CMV infections in the immunocompromised host. This compound is a potent inhibitor of poxvirus replication in vitro (9) and has been shown to be very effective against both VV and CV infections in animal models, including in immunocompromised mice (2, 4, 22, 26, 27). Although there are other phosphonate nucleotides and their analogs, such as adefovir and adefovir dipivoxil, that are currently under evaluation in clinical studies for treatment of human immunodeficiency virus and/or hepatitis virus infections, there is far less information regarding their activity against poxvirus infections than is available for CDV (20).
Although CDV is approved for intravenous use in serious CMV infections in AIDS patients, there are a number of problems associated with its use. Two major problems that limit its use are its nephrotoxicity (13, 25) and its poor oral bioavailability. The toxicity of CDV may not be a limiting factor for its use during a poxvirus outbreak, as treatment would be expected to be of short duration. Additionally, since the drug has a long intracellular half-life, dosing can be infrequent, i.e., once or twice per week (2, 6, 19). Its lack of oral activity, however, would present much greater logistical problems, as the drug would need to be administered intravenously. A number of analogs of the phosphonate nucleotides, including cCDV and adefovir dipivoxil, have been synthesized to increase the oral bioavailability of these compounds. Another approach to improving the oral bioavailability of nucleosides has been to add certain ether lipid groups to increase oral absorption and cell membrane penetration. Members of our group have had previous experience utilizing this technology for improving the activity of nucleosides such as acyclovir, ganciclovir, penciclovir, and azidothymidine (14-16).
Earlier studies indicated that 1-0-hexadecylpropanediol-P-ganciclovir provided greater oral bioavailability in mice than ganciclovir and provided good oral activity against herpes simplex virus type 1 and murine CMV infections in mice (16). In addition, it was demonstrated that oral 1-0-hexadecyl-propanediol-P-acyclovir at 20 mg/kg of body weight daily lowered woodchuck hepatitis virus DNA levels by nearly 2 log units after 4 weeks of administration. Acyclovir at a fivefold-higher molar dose was not effective (15). To determine if a similar strategy could be employed with phosphonate nucleotides, we synthesized the 1-0-hexadecylpropanediol and the 1-0-octadecylethanediol esters of CDV and cCDV. As demonstrated in these studies, the new analogs were not only active against VV and CV replication in vitro, they were considerably more active than the parent compounds. Although there are no published reports of the efficacy of CDV for experimental variola virus infections, it was recently determined that CDV and cCDV inhibited the replication of variola virus strain Bangladesh in vitro at levels of about 25 μM. Importantly, the alkoxyalkyl analogs of CDV and cCDV were >100-fold more active than the unmodified compounds (John Huggins, unpublished results). The mechanism of action through which these analogs exhibit greater antiviral activity and selectivity is currently not well understood. However, preliminary studies using radiolabeled HDP-[14C]CDV, have indicated that cellular uptake of the drug is many-fold greater than that observed with [14C]CDV in human lung fibroblast cells (unpublished observations).
In summary, we have synthesized several alkoxyalkyl esters of CDV and cCDV which exhibited greatly enhanced antiviral activity and selectivity against two members of the orthopoxvirus group in tissue culture cells. Based upon our previous experiments with analogs of this type, we anticipate that these new compounds will exhibit significant oral activity compared with the parent compounds, and they now need to be evaluated in animal model systems for oral pharmacokinetics, toxicity, and antiviral efficacy against members of the orthopoxviruses.
Acknowledgments
These studies were supported in part by NIH grant EY11832 from the National Eye Institute to the University of California, San Diego (K.Y.H.), and contract NO1-AI-85347 from the Antiviral Research Branch, NIAID, NIH, to the University of Alabama at Birmingham (E.R.K.).
REFERENCES
- 1.Bischofberger, N., M. J. M. Hitchcock, M. S. Chen, D. B. Barkhimer, K. C. Cundy, K. M. Kent, S. A. Lacy, W. A. Lee, Z.-H. Li, D. B. Mendel, D. F. Smee, and J. L. Smith. 1994. 1-[((s)-2-Hydroxy-2-oxo-1,4,2-dioxaphosphorinan-5-yl)methyl]cytosine, an intracellular prodrug for (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine, with improved therapeutic index in vivo. Antimicrob. Agents Chemother. 38:2387-2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray, M., M. Martinez, D. F. Smee, D. Kefauver, E. Thompson, and J. W. Huggins. 2000. Cidofovir protects mice against lethal aerosol or intranasal cowpox virus challenges. J. Infect. Dis. 181:10-19. [DOI] [PubMed] [Google Scholar]
- 3.Breman, J. G., and D. A. Henderson. 1998. Poxvirus dilemmas--monkeypox, smallpox, and biological terrorism. N. Engl. J. Med. 339:556-559. [DOI] [PubMed] [Google Scholar]
- 4.Collins, D. J., D. C. Quenelle, and E. R. Kern. 2001. Systemic and cutaneous infections of mice with vaccinia and cowpox viruses and efficacy of cidofovir. Antiviral Res. 50:A70.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cundy, K. C., A. M. Bidgood, G. Lynch, J.-P. Shaw, L. Griffin, and W. A. Lee. 1996. Pharmacokinetics, bioavailability, metabolism and tissue distribution of cidofovir (HPMPC) and cyclic HPMPC in rats. Drug Metab. Disp. 24:745-752. [PubMed] [Google Scholar]
- 6.Cundy, K. C., Z.-H. Li, M. J. M. Hitchcock, and W. A. Lee. 1996. Pharmacokinetics of cidofovir in monkeys. Evidence for a prolonged elimination phase representing phosphorylated drug. Drug Metab. Disp. 24:734-744. [PubMed] [Google Scholar]
- 7.De Clercq, E., M. Luczak, D. Shugar, P. F. Torrence, J. A. Waters, and B. Witkop. 1976. Effect of cytosine arabinoside, iododeoxyuridine, ethyldeoxyuridine, thiocyanatodeoxyuridine, and ribavirin on tail lesion formation in mice infected with vaccinia virus. Proc. Soc. Exp. Biol. Med. 151:487-490. [DOI] [PubMed] [Google Scholar]
- 8.De Clercq, E., T. Sakuma, M. Baba, R. Pauwels, J. Balzarini, I. Rosenberg, and A. Holý. 1987. Antiviral activity of phosphonylmethoxyalkyl derivatives of purine and pyrimidines. Antiviral Res. 8:261-272. [DOI] [PubMed] [Google Scholar]
- 9.De Clercq, E. 2001. Vaccinia virus inhibitors as a paradigm for the chemotherapy of poxvirus infections. Clin. Microbiol. Rev. 14:382-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fenner, F., R. Wittek, and K. R. Dumbell. 1986. The orthopoxviruses. Academic Press, New York, N.Y.
- 11.Henderson, D. A. 1998. Bioterrorism as a public health threat. Emerg. Infect. Dis. 4:488-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heymann, D. L., M. Szczeniowski, and K. Esteves. 1998. Re-emergence of monkeypox in Africa: a review of the past six years. Br. Med. Bull. 54:693-702. [DOI] [PubMed] [Google Scholar]
- 13.Hitchcock, M. J. M., H. S. Jaffe, J. C. Martin, and R. J. Stagg. 1996. Cidofovir, a new agent with potent anti-herpesvirus activity. Antivir. Chem. Chemother. 7:115-127. [Google Scholar]
- 14.Hostetler, K. Y., J. R. Beadle, G. D. Kini, M. F. Gardner, K. N. Wright, T.-H. Wu, and B. E. Korba. 1997. Enhanced oral absorption and antiviral activity of 1-O-octadecyl-sn-glycero-3-phospho-acyclovir in hepatitis B virus infection, in vitro. Biochem. Pharmacol. 53:1815-1822. [DOI] [PubMed] [Google Scholar]
- 15.Hostetler, K. Y., J. R. Beadle, W. E. Hornbuckle, C. A. Bellezza, I. A. Tochkov, P. J. Cote, J. L. Gerin, B. E. Korba, and B. C. Tennant. 2000. Antiviral activities of oral 1-O-hexadecylpropanediol-3-phosphoacyclovir and acyclovir in woodchucks with chronic woodchuck hepatitis virus infection. Antimicrob. Agents Chemother. 44:1964-1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hostetler, K. Y., R. J. Rybak, J. R. Beadle, M. F. Gardner, K. A. Aldern, K. N. Wright, and E. R. Kern. 2001. In vitro and in vivo activity of 1-O-hexadecylpropanediol-3-phospho-ganciclovir and 1-O-hexadecylpropanediol-3-phospho-penciclovir in cytomegalovirus and herpes simplex virus infections. Antivir. Chem. Chemother. 12:61-70. [DOI] [PubMed] [Google Scholar]
- 17.Hutin, Y. J., R. J. Williams, P. Malfait, R. Pebody, V. N. Loparev, S. L. Ropp, M. Rodriguez, J. C. Knight, F. K. Tshioko, A. S. Khan, M. V. Szczeniowski, and J. J. Esposito. 2001. Outbreak of human monkeypox, Democratic Republic of Congo, 1996-1997. Emerg. Infect. Dis. 7:434-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jezek, Z., M. Szczeniowski, K. M. Paluku, and M. Mutombo. 1987. Human monkeypox: clinical features of 282 patients. J. Infect. Dis. 156:293-298. [DOI] [PubMed] [Google Scholar]
- 19.Kern, E. R. 1991. Value of animal models to evaluate agents with potential activity against human cytomegalovirus. Transplant Proc. 23:152-155. [PubMed] [Google Scholar]
- 20.Naesens, L., R. Snoeck, G. Andrei, J. Balzarini, J. Neyts, and E. De Clercq. 1997. HPMPC (cidofovir), PMEA (adefovir) and related acyclic nucleoside phosphonate analogues: a review of their pharmacology and clinical potential in the treatment of viral infections. Antivir. Chem. Chemother. 8:1-23. [Google Scholar]
- 21.Nettleton, P. F., J. A. Gilray, H. W. Reid, and A. A. Mercer. 2000. Parapoxviruses are strongly inhibited in vitro by cidofovir. Antiviral Res. 48:205-208. [DOI] [PubMed] [Google Scholar]
- 22.Neyts, J., and E. De Clercq. 1993. Efficacy of (S)-1-(3-Hydroxy-2-Phosphonylmethoxypropyl) cytosine for the treatment of lethal vaccinia virus infections in severe combined immune deficiency (SCID) mice. J. Med. Virol. 41:242-246. [DOI] [PubMed] [Google Scholar]
- 23.Neyts, J., and E. De Clercq. 2001. Efficacy of 2-amino-7-(1,3-dihydroxy-2-propoxymethyl) purine for treatment of vaccinia virus (orthopoxvirus) infections in mice. Antimicrob. Agents Chemother. 45:84-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Toole, T. 1999. Smallpox: an attack scenario. Emerg. Infect. Dis. 5:540-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Safrin, S., J. Cherrington, and H. S. Jaffe. 1997. Clinical uses of Cidofovir. Rev. Med. Virol. 7:145-156. [DOI] [PubMed] [Google Scholar]
- 26.Smee, D. F., K. W. Bailey, M.-H. Wong, and R. W. Sidwell. 2000. Intranasal treatment of cowpox virus respiratory infections in mice with cidofovir. Antiviral Res. 47:171-177. [DOI] [PubMed] [Google Scholar]
- 27.Smee, D. F., K. W. Bailey, and R. W. Sidwell. 2001. Treatment of lethal vaccinia virus respiratory infections in mice with cidofovir. Antivir. Chem. Chemother. 12:71-76. [DOI] [PubMed] [Google Scholar]