Abstract
The 13-residue dermaseptin S4 derivative K4S4(1-13)a (P) was previously shown to kill intraerythrocytic malaria parasites through the lysis of the host cells. In this study, we have sought peptides that will kill the parasite without lysing the erythrocyte. To produce such peptides, 26 compounds of variable structure and size were attached to the N terminus of P and screened for antiplasmodium and hemolytic activities in cultures of Plasmodium falciparum. Results from this screen indicated that increased hydrophobicity results in amplified antiplasmodium effect, irrespective of the linearity or bulkiness of the additive. However, increased hydrophobicity also was generally associated with increased hemolysis, with the exception of two derivatives: propionyl-P (C3-P) and isobutyryl-P (iC4-P). Both acyl-peptides were more effective than P, with 50% growth inhibition at 3.8, 4.3, and 7.7 μM, respectively. The antiparasitic effect was time dependent and totally irreversible, implying a cytotoxic effect. The peptides were also investigated in parallel for their ability to inhibit parasite growth and to induce hemolysis in infected and uninfected erythrocytes. Whereas the dose dependence of growth inhibition and hemolysis of infected cells overlapped when cells were treated with P, the acyl-peptides exerted 50% growth inhibition at concentrations that did not cause hemolysis. Noticeably, the acyl derivatives, but not P, were able to dissipate the parasite plasma membrane potential and cause depletion of intraparasite potassium under nonhemolytic conditions. These results clearly demonstrate that the acyl-peptides can affect parasite viability in a manner that is dissociated from lysis of the host cell. Overall, the data indicate the potential usefulness of this strategy for development of selective peptides as investigative tools and eventually as antimalarial agents.
Malaria still constitutes the most widespread infectious disease, affecting hundreds of millions of people, mostly in Third World countries, and causing the death of one million children every year in Africa alone (40). This devastating situation stems from the ever-increasing resistance of parasites to available drugs, and new chemotherapeutic means have to be developed. Recently, various animal-derived peptide-based antimicrobials have emerged as interesting tools for exploring new potential antiplasmodiams agents (8, 12, 13).
Antimicrobial peptides are generally made up of less than 50 amino acids, with an excess of basic amino acids (lysine and/or arginine) over acidic residues and a substantial portion of hydrophobic amino acids (3, 7, 10, 17, 27). They vary considerably in structure, size, amino acid sequence, and spectrum of action. Structure-activity relationship studies have shown that the most potent peptides are highly basic, with a pronounced amphipathic character (2, 4, 18, 24, 26, 35). These peptides exert antimicrobial action through their effect on the membrane of target cells by a mechanism the details of which remain to be understood. From available information, it can be suggested that they don't interact with stereospecific targets, such as protein receptors, enzymes, and so on (5, 38). Their charge and hydrophobicity are the main factors affecting antimicrobial activity. The strong sigmoid nature of their dose-response effect (25, 33) suggests that the active form of the peptide is an oligomer. Some of these peptides were stipulated to form ion channels or pores, as suggested by their ability to dissipate electric potential across energy-transducing membranes and ion conductance across lipid bilayers (32, 41). Various basic models for a membranolytic mechanism were proposed, ranging from pore formation to induction of structural defects (11, 21, 28, 30, 36, 39). The perturbations in the membrane structure lead to membrane permeabilization. Hence, essential ions and metabolites are free to leak in and out and dissipate electric potential across the membrane. This stops ATP production and arrests cellular metabolism, eventually leading to cell death.
Many antimicrobial peptides display a broad spectrum of activity that can kill or neutralize gram-negative and -positive bacteria, yeast and filamentous fungi, and some enveloped viruses, as well as many types of cancer cells (19, 23, 31). Yet, many of these peptides are quite inactive on normal eukaryotic cells. Although the basis for this discrimination is also unclear, it appears to be related to the lipid composition of the target membrane (i.e., fluidity, negative charge density, and absence or presence of cholesterol) and the presence in the peptide-susceptible organisms of a large negative transmembrane electrical potential (1). Thus, while the precise mechanism of action of antimicrobial peptides is yet to be better defined, microbicidal effect is widely believed to result from their capacity to permeabilize the membrane of target cells. Such a mechanism of action endows the peptide-based antimicrobial system with attractive advantages over classical antibiotics, because it makes it extremely difficult for microbes to develop resistance (4, 15, 22). However, a major downside of such a mechanism is reflected in its unselective activity over a wide range of cell types, which could be problematic—for instance, in systemic routes of administration (9). In an ironic way, therefore, a major challenge of this field of research consists of figuring out how to endow specificity to a system that, by definition, is nonspecific.
The hemolytic antimicrobial peptide dermaseptin S4 has been shown before to exert antiplasmodium activity (8). Subsequent studies (5, 6, 13) with derivatives of S4 against Plasmodium falciparum, the most lethal human parasite, identified K4K20-S4 as the most potent peptide (50% inhibitory concentration [IC50] = 0.2 μM), with its shorter version, K4-S4(1-13)a, displaying considerable effectiveness (IC50 = 6 μM). Both peptides acted more efficiently at the trophozoite stage. The antiplasmodium action was rapid and mediated by the lysis of the infected erythrocyte. Since these peptides were also lytic to normal erythrocytes, albeit at a 30-fold-higher concentration, it was deemed necessary to develop additional derivatives that could affect the parasite with minimal threat to erythrocytes. In this study, we investigated the antiplasmodium activities of various new dermaseptin S4 derivatives. We have synthesized a peptide library based on the 13-residue dermaseptin S4 derivative K4-S4(1-13), and after screening for the most effective compounds, we investigated their detailed mechanism of antiplasmodium action.
MATERIALS AND METHODS
Preparation of dermaseptin S4 derivatives.
The reference peptide K4-S4(1-13) was synthesized by the solid-phase method as described previously (5), applying the Fmoc (9-fluorenylmethyloxycarbonyl) active ester chemistry on a fully automated, programmable Applied Biosystems model 433A peptide synthesizer (Applied Biosystems). MBHA resin (Novabiochem, Darmstadt, Germany) was used to obtain an amidated peptide. Unless otherwise stated, all reagents were purchased from Sigma (St. Louis, Mo.). The various analogs were prepared by linking the N terminus of K4-S4(1-13) to one of the compounds detailed in Table 1. Selective reaction with the amino-terminal group was ensured by selective deprotection (removal of Fmoc from the N terminus) of the Fmoc-protected resin-bound peptide by mixing the resin with a solution of 20% piperidine-N-methylpyrrolidone (NMP), whereas all other potentially reactive groups remained masked by orthogonal protecting groups.
TABLE 1.
Structure of the parent peptide K4-S4(1-13)a (P) and its derivatives
| Designation | Peptide | Bond typea | % Acetonitrileb |
|---|---|---|---|
| P | ALWKTLLKKVLKA-CONH2c | Amine | 45 |
| Derivatives (group I) | |||
| tM∼P | Trimethyl-P | Alkylamine | 47 |
| Mal-P | Malonyl-P | Amide | 51 |
| Suc-P | Succinyl-P | Amide | 52 |
| tME-Suc-P | Trimethylethylamino-succinyl-P | Amide | 56 |
| C4-Suc-P | Butylamino-succinyl-P | Amide | 57 |
| C6-Suc-P | Hexylamino-succinyl-P | Amide | 61 |
| C12-Suc-P | Dodecylamino-succinyl-P | Amide | 66 |
| C8=P | Octylsulfonyl-P | Sulfonamide | 60 |
| cHA-P | Cyclohexylacetyl-P | Amide | 58 |
| Bz-P | Benzoyl-P | Amide | 56 |
| Bz-Suc-P | Benzylamino-succinyl-P | Amide | 57 |
| Fmoc-P | N, 9, Fluorenylmethyloxycarbonyl-P | Amide | 64 |
| Derivatives (group II) | |||
| C2-P | Acetyl-P | Amide | 53 |
| C3-P | Propionyl-P | Amide | 53.5 |
| C4-P | Butyryl-P | Amide | 54 |
| C5-P | Pentanoyl-P | Amide | 55 |
| C6-P | Hexanoyl-P | Amide | 57 |
| C8-P | Octanoyl-P | Amide | 60 |
| C10-P | Decanoyl-P | Amide | 63 |
| C12-P | Dodecanoyl-P | Amide | 64 |
| C16-P | Hexadecanoyl-P | Amide | 69 |
| Derivatives (group III) | |||
| C2 P | Ethylsulfonyl-P | Sulfonamide | 53 |
| C4 P | Butylsulfonyl-P | Sulfonamide | 54 |
| OC4-P | Hydroxybutyryl-P | Amide | 52 |
| SC3-P | Mercaptopropionyl-P | Amide | 54 |
| iC4-P | Isobutyryl-P | Amide | 54 |
Bond type at the N terminus of the parent peptide is represented by ∼ for alkylamine, - for amide, and for sulfonamide.
Indication for hydrophobicity. Shown is the percent acetonitrile (in a mixture of acetonitrile and water) at which peptide was eluted with a linear gradient (1%/min) and a C4 column in reversed-phase HPLC.
Amino acid sequence in the one-letter code.
For amide-linked derivatives, the deprotected resin-bound peptide (20 mg) was washed with NMP to remove the piperidine and suspended in 0.7 ml of dimethylformamide (DMF), to which a twofold molar excess of the relevant acid compound was added followed by threefold molar excess of 1-ethyl-3-(dimethylaminopropyl)carbodiimide (EDAC). Using the same chemistry, part of the succinyl derivative was reactivated to allow additional linkages to various amines, as detailed in Table 1. For sulfonamide derivatives, the deprotected resin-bound peptide was suspended in 0.7 ml of anhydrous DMF, to which was added a 1.5-fold molar excess of the relevant alkylsulfonyl chloride derivative, followed by 2-fold molar excess of diisopropylethylamine (DIPEA). For trimethyl-P synthesis the deprotected resin-bound peptide was suspended in 1 ml of DMF-methanol (1:4), to which was added a 10-fold molar excess of both methyliodide and potassium carbonate.
The reaction mixtures were sonicated (5 min) and then agitated for 24 h at room temperature. Each mixture was then centrifuged for 3 min (supernatant discarded), and the resin was washed four times with DMF and then three times with ether-dichloromethane (1:1). The residue was dried for 1 h at room temperature and then 4 h at 50°C.
Peptide cleavage from the resin was performed in a mixture of 2.5% water, 2.5% triethylsilane, and 95% trifluoroacetic acid (TFA), and stirred in an ice bath for 15 min and then at room temperature for 2 h. After filtration of the resin, the peptide-TFA filtrate was precipitated (added drop by drop) in ice-cold diethyl ether and placed in a fume hood to dry at 60°C for 2 h and then dissolved in 10% acetic acid and lyophilized. The crude peptide was purified to chromatographic homogeneity in the range of 95 to >99% by reverse-phase high-performance liquid chromatography (HPLC) (Alliance-Waters). HPLC runs were performed on a semipreparative C4 column (Vydac) with a linear gradient of acetonitrile in water (1%/min); both solvents contained 0.1% TFA. Based on peak integration of the HPLC profiles, the yield for each amide or sulfonamide linkage was generally not lower than 85%, with the exception of C16-P, where the yield was ∼65%. The yield for tM∼P was 63%.
The purified peptides were subjected to amino acid analysis and mass spectrometry in order to confirm their composition. Peptides were stocked as lyophilized powder at −20°C. Prior to testing, fresh solutions were prepared in water, briefly vortexed, sonicated, centrifuged, and then diluted in the appropriate medium. Buffers were prepared with distilled water (mQ; Millipore). All other reagents were analytical grade.
Parasite cultivation.
The W2 strain of P. falciparum was used throughout this investigation with human erythrocytes (RBCs). Parasites were cultivated by the Jensen and Trager technique as modified in our laboratory (14). Culture was synchronized by the sorbitol method (16) with the less toxic alanine, and infected cells were enriched from culture by Percoll-alanine gradient centrifugation (14).
Drug screening test.
Synchronized cultures at the ring stage were cultured at 1% hematocrit and 2% parasitemia in the presence of 10 μM dermaseptin derivatives. After 18 h of incubation, the cultures were divided into two sets. [3H]hypoxanthine (final concentration, 2 μCi/ml) was added to one set, and cells were harvested 6 h later. The cell-associated radioactivity was determined, and inhibition of growth was calculated by comparison with that of controls (without peptide). The second set was further incubated for 6 h, and the concentration of hemoglobin in the supernatant was determined by absorption spectroscopy at 405 nm. Full lysis was obtained by lysis of the same number of cells in an equal volume of distilled water.
Determination of IC50.
Synchronized cultures at the ring stage were cultured at 1% hematocrit and 2% parasitemia in the presence of increasing concentrations of lipodermaseptin derivatives. After 18 h of incubation, parasite viability was determined by the hypoxanthine incorporation test. The IC50 was determined by nonlinear regression fitting of the data with the Sigmaplot program. By using the same technique for the measurement of parasite viability and hemolysis, the stage and the time dependence of drug effect at each of the different stages was also determined. The precise conditions are shown in the legends to the relevant figures in the Results section.
Dissipation of parasite membrane potential by the acylated derivatives.
Whole culture at the trophozoite stage in modified growth medium (wash medium containing 10 mM bicarbonate and 7% plasma) at 0.5% hematocrit was incubated in the presence of 1 μM rhodamine 123 (R123) for 30 min at 37°C. R123 accumulates inside cells in proportion to the membrane potential (ΔΨ) and has been shown to respond to the dissipation of the plasma membrane ΔΨ in P. falciparum (34). Aliquots of this culture were then exposed to different peptides or to a mixture of 10 μM nigericin (K+:H+ exchanger) and 10 μM monensin (Na+:H+ exchanger) to dissipate the ion gradient across membranes (positive control). At different time intervals, samples were taken, and cells were pelleted by centrifugation, washed in phosphate-buffered saline (PBS), and resuspended in the original sample volume of PBS. Aliquots of 120 μl were placed in a 96-well plate and read in a fluorescence reader (excitation wavelength [λex] = 530 nm, emission wavelength [λem] = 585 nm). Relative fluorescence (as a percentage of that of the untreated control at the same time) was plotted against the time of incubation.
Depletion of the parasite's intracellular potassium in the presence of dermaseptin derivatives.
Infected cells at the young trophozoite stage were separated from uninfected RBCs with the Percoll-alanine gradient and incubated for different time intervals in culture medium at 37°C. Infected cells (∼97% parasitemia, determined on Giemsa-stained thin blood smears) were suspended at 0.5% hematocrit in culture medium containing 15 μM dermaseptin derivatives. At time zero and at 4 h, aliquots were taken, and cells were pelleted by centrifugation, washed in PBS, and centrifuged again. To obtain free parasite, cells were resuspended in PBS containing 0.003% (wt/vol) saponin and after several minutes at room temperature were pelleted by centrifugation, washed in PBS several times, and finally washed with 110 mM MgCl2 buffered with 10 mM HEPES. After disruption of the free parasites by freezing and thawing and centrifugation at 10,000 × g for 20 min, the supernatant liquid was analyzed for potassium content by inductive-coupled plasma-atomic emission spectroscopy on an Optima 3300 ICP-AES system (Perkin-Elmer, Norwalk, Conn.). Relative intracellular potassium concentrations were calculated as a percentage of that of the untreated control at the same time.
RESULTS
We have shown previously that dermaseptins and their derivatives are able to kill intraerythrocytic malaria parasites (13). This effect was demonstrably due to the lysis of the infected cell. Uninfected cells were similarly lysed, albeit at higher peptide concentrations, but such differential lysis was insufficient to ensure safe therapy with no threat to the host. We have therefore sought peptides that will kill the parasite without lysing the erythrocyte. To produce such peptides, 25 compounds of variable structure and size were attached to the N terminus of K4-S4(1-13)a. The resulting peptide derivatives are shown in Table 1.
Screen of antiplasmodium and hemolytic activities.
Results shown in Fig. 1 for group I indicate that various derivatives of K4-S4(1-13)a have pronounced antiplasmodium activity. Compared to the parent peptide, this series of compounds includes derivatives with increased positive charge at the N terminus (tM∼P), increased negative charge (Mal-P and Suc-P), and increased hydrophobicity, both linear (tME-Suc-P, C4-Suc-P, C6-Suc-P C12-Suc-P, and C8=P) and cyclic (cHA-P, Bz-P, Bz-Suc-P, and Fmoc-P).
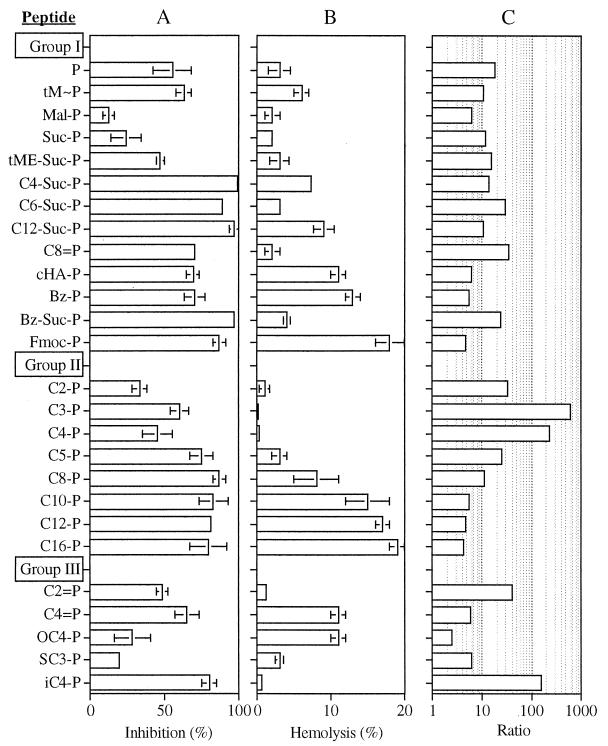
FIG. 1.
Screen of antiplasmodium and hemolytic activities for three groups of peptides. Synchronized cultures of infected human RBCs at the ring stage were cultured at 1% hematocrit and 2% parasitemia in the presence of a 10 μM concentration of each of the designated peptides (Table 1). After 18 h of incubation, the cultures were divided into two sets. [3H]hypoxanthine was added to one set, and cells were harvested 6 h later. The cell-associated radioactivity was determined, and inhibition of growth was calculated by comparison with that of controls (A). The second set was further incubated for 6 h, and the concentration of hemoglobin in the supernatant was determined by absorption spectroscopy at 405 nm. Hemolysis was calculated by comparison with fully hemolyzed cultures (B). Error bars represent the standard deviation from the mean, calculated from at least two independent experiments performed in quadruplicates. Panel C shows the ratio (A/B) calculated from the mean values from panels A and B. Note the logarithmic scale of the ratio axis.
From the antiplasmodium effect (Fig. 1A), we can conclude that increased hydrophobicity results in an amplified effect, irrespective of the linearity or bulkiness of the additive. Derivatives that increased the peptide's negative charge reduced the antiplasmodium effect, whereas the positive charge in tM∼P did not substantially improve growth inhibition. Increased hydrophobicity, however, also was generally associated with increased hemolysis (Fig. 1B), with the exception of two derivatives: Bz-Suc-P and C8=P. We have chosen to compare the ratio of relative inhibition to relative hemolysis as a selection criterion for the most suitable derivative. This analysis (Fig. 1C) revealed that Bz-Suc-P and C8=P were the most selective derivatives: compared to the parent peptide, the ratio was up to twofold higher.
Since hydrophobicity emerged from this screen as a suitable property, we have screened a second series of compounds in which the length of the acyl chain was systematically increased from acetyl (C2-P) to palmitoyl (C16-P). Results shown in Fig. 1 for group II confirmed this notion, because the antiplasmodium activity increased with chain length (Fig. 1A). This was not the case for the hemolytic activity (Fig. 1B). Increasing the length from C2 up to C4 resulted in decreased activity, whereas a further increase in length led to a gradual increase in hemolysis. With the ratio criterion for selection of optimal compounds in this series, it can be seen that C3-P and C4-P were at the top of the list.
Following the results obtained with the first two series of compounds, we have screened a third series that consisted of various C2-C4 derivatives (group III). In this series, which included two sulfonamide and three amide-linked acyl derivatives, we have attempted to further modulate N-terminal hydrophobicity, assuming that this will reduce the hemolytic activity. Two derivatives (iC4-P and C4=P) exhibited increased antiplasmodium activity compared to the parent peptide (Fig. 1A), but only iC4-P displayed both increased antiplasmodium effect and decreased hemolysis (Fig. 1B). The calculated ratio (Fig. 1C) was the highest obtained in this series of compounds and was therefore selected along with C3-P from the second series for further and more detailed investigations.
Detailed determination of antiplasmodium activity and stage dependence of selected compounds.
The dose response of the two acyl-peptides C3-P and iC4-P was investigated and compared to that of the parent peptide, P, by using synchronized cultures that were exposed to the peptides either at the ring stage or at the trophozoite stage. Both acyl-peptides were more effective than P at the ring stage, but not at the trophozoite stage (Table 2). The stage dependence results indicated that ring-stage parasites were somewhat less sensitive than the more mature trophozoite stage. This was essentially found with P, as previously observed (13), but much less with iC4-P and not at all with C3-P. The slopes of the dose-response curves varied with peptide and stage, indicating some fine differences in the stoichiometry of drug and target relations.
TABLE 2.
Stage dependence of the growth inhibitory activity of the parent peptide K4S4(1-13)a (P) and two derivatives
| Peptide | Mean IC50 ± SE (μM)a |
Slope |
||
|---|---|---|---|---|
| Ring | Trophozoite | Ring | Trophozoite | |
| P | 7.66 ± 0.85 | 3.35 ± 0.25 | 1.47 ± 0.20 | 1.44 ± 0.12 |
| C3-P | 4.33 ± 0.25 | 3.80 ± 0.32 | 2.23 ± 0.26 | 1.64 ± 0.12 |
| iC4-P | 3.82 ± 0.47 | 2.54 ± 0.34 | 1.79 ± 0.37 | 1.29 ± 0.20 |
IC50 represents the peptide concentration that produced 50% inhibition of hypoxanthine uptake after 18 h in culture. The standard error (SE) was calculated from at least two independent experiments performed in quadruplicates.
Time dependence and reversibility of antiplasmodial action.
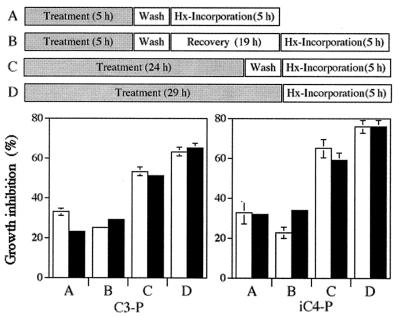
The time dependence of the antiplasmodium effect of the two acyl-peptides was investigated by using synchronized culture at the ring or at the trophozoite stage. Parasites were exposed to either C3-P or iC4-P at 10 μM for various lengths of time. Thereafter, the peptides were washed, and the cultures were tested for parasite viability either immediately or after a period of recovery under the culture conditions. The precise protocols are shown in the upper part of Fig. 2. Less inhibition after a longer recovery time in the absence of peptides indicates reversibility of drug action (a cytostatic effect), whereas when no change in parasite viability is observed due to removal of the peptide, an irreversible or cytotoxic effect is the cause.
FIG. 2.
Stage dependence of antiplasmodium activity. Infected cells at the ring or at the trophozoite stage (1% hematocrit and 2% parasitemia) were exposed to either C3-P or iC4-P (10 μM), and parasite growth was determined by hypoxanthine (Hx) incorporation. The various protocols for time of exposure to the peptides, recovery without peptide, and hypoxanthine incorporation (A to D) are depicted in the upper part. Open bars, ring stage; solid bars, trophozoite stage. Each experiment was performed twice in triplicates. Error bars represent the standard deviation from the mean.
As can be seen from Fig. 2, drug action is time dependent. Inhibition of parasite growth increased with the time of exposure to the peptide, reaching above 50% inhibition in ≈24 h. This time course of peptide action does not depend on the parasite stage that was exposed to the drug. Allowing the parasites to recover under culture conditions did not restore their viability, except for slightly reduced inhibition after recovery in the ring-stage parasites after 5 h of treatment with acyl-peptides. Thus, inhibition of parasite growth was essentially irreversible, implying a cytotoxic effect.
Hemolytic activity versus antiplasmodium activity.
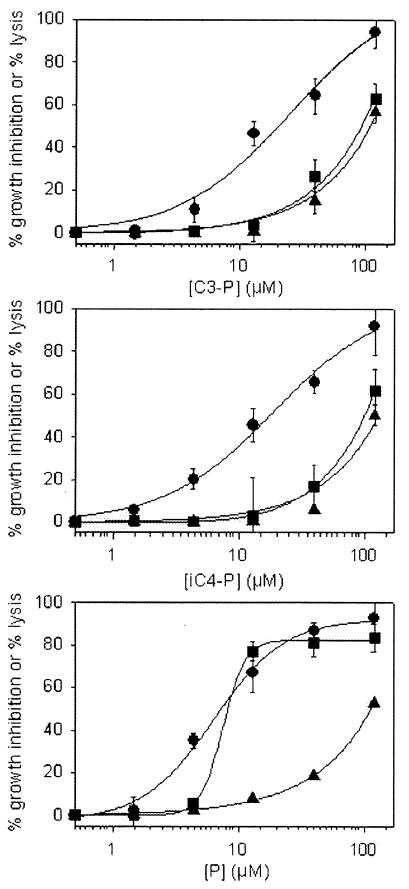
To elucidate the mechanism of antiplasmodium activity, C3-P and iC4-P were investigated simultaneously for their ability to induce hemolysis and to inhibit parasite growth. Infected cells at the young trophozoite stage were separated from normal RBCs by the Percoll-alanine gradient method. Infected (≥90% parasitemia) and uninfected cells were resuspended in culture medium (0.5% hematocrit) containing increasing concentrations of the peptides. After 2 h of incubation, [3H]hypoxanthine was added to one set of culture, and cells were harvested 4 h later. Hemolysis was determined in two additional sets of cultured infected and normal RBCs by measuring the specific absorbance of hemoglobin in the supernatants after 6 h of exposure to acylated peptides. C3-P (Fig. 3A) and iC4-P (Fig. 3B) were slightly more inhibitory to parasite growth than P was (Fig. 3C). Under these conditions of brief exposure of trophozoite-enriched cultures, the IC50 were essentially equal: 19 ± 4.4 and 21 ± 7.5 μM, respectively, for C3-P and iC4-P and 19.6 ± 1.6 μM for P.
FIG. 3.
Hemolytic activity versus antiplasmodium activity. Infected cells at the young trophozoite stage were separated from normal RBCs by the Percoll-alanine gradient method. Infected (≥90% parasitemia) and uninfected cells were resuspended in culture medium (0.5% hematocrit) containing increasing concentrations of the peptides. After 2 h of incubation, hypoxanthine was added to one set of the culture, cells were harvested 4 h later, and the cell-associated radioactivity was determined. Hemolysis was determined in two additional sets of cultured infected and normal RBCs by measuring the specific absorbance of hemoglobin in the supernatants after 6 h of exposure to the peptides. The continuous lines depict the best fit of the data to the dose response. •, inhibition of hypoxanthine uptake; ▪, hemolysis of infected RBCs; ▴, hemolysis of normal RBCs. Each experiment was performed at least twice in quadruplicates. Error bars represent the standard deviation from the mean. If no error bar is shown, the standard deviation was smaller than the diameter of the symbol.
Dissipation of the parasite plasma membrane potential.
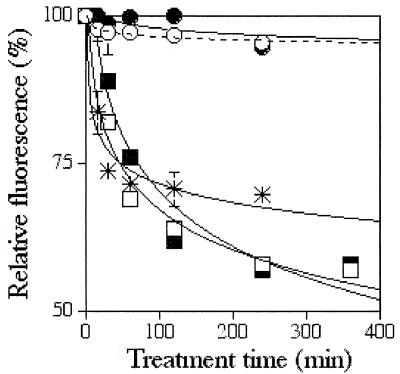
To assess whether the peptide can gain access to the parasite membrane, the dissipation of R123 fluorescence was monitored. This fluorescent dye accumulates in parasites in correlation with the parasite membrane ΔΨ. It was first ascertained that fluorescence measurement does indeed dissipate ΔΨ as measured by reduced accumulation of the dye with the ionophores nigericin and monensin (Fig. 4). It was subsequently found that both C3-P and iC4-P depolarized the parasite membrane in a time-dependent manner, whereas P did not. These results were obtained in the presence of 10 μM acyl-peptides: i.e., at ∼2.5-fold the IC50 of the acyl-peptides, where hemolysis was minimal. At this concentration, P neither killed the parasite nor lysed the infected cells. These results clearly demonstrate that the peptide can cross the host cell membrane and interact with the parasite plasma membrane to permeabilize it. As we saw previously, there was no significant difference between the potency of the two acyl-peptides to elicit this effect.
FIG. 4.
Dissipation of the parasite plasma membrane potential. Trophozoites that had been preincubated with R123 were exposed to one of the designated peptides and to a mixture of known ionophores (monensin and nigericin). Samples were taken at different time intervals, washed, and resuspended in the original sample volume of PBS. The fluorescence was read (λex = 530 nm, λem = 585 nm). Relative fluorescence (as percentage of untreated control at the same time) was plotted against the time of exposure. ○, PBS; •, P; □, C3-P; ▪, iC4-P; ✳, nigericin plus monensin. Each experiment was performed twice in duplicates. Error bars represent the standard deviation from the mean. If no error bar is shown, the standard deviation was smaller than the diameter of the symbol.
Measurement of intracellular potassium concentration of the parasite by atomic emission.
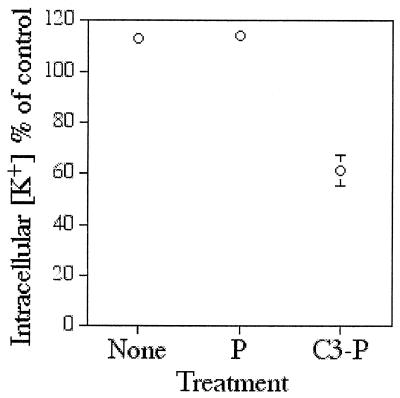
Changes in the parasite's intracellular potassium concentration were investigated in the presence of P and C3-P. (Only one acyl-peptide was examined, since so far, its action had been found to be almost superimposed with that of iC4-P.) Infected cells at the young trophozoite stage were separated from normal RBCs by using Percoll-alanine gradient. Infected cells (∼90% parasitemia) were resuspended in culture medium containing 10 μM P or C3-P. After 4 h of incubation, the cell parasites were freed from their host cell by saponin lysis and washed extensively to remove host cell material and finally in MgCl2. Free parasites were disrupted by freezing and thawing, and the potassium content in the supernatant was determined. The results depicted in Fig. 5 clearly indicate that treatment with C3-P, but not with P, resulted in marked depletion of parasite potassium. This adds to the previous demonstration that the acyl-peptide is able to cross the host cell membrane and interact with that of the parasite to permeabilize it.
FIG. 5.
Measurement of potassium intracellular concentration of the parasite. Infected cells at the young trophozoite stage (∼97% parasitemia) were cultured in the absence or presence of peptide (10 μM). After 4 h of incubation, parasites were freed from their host cell by saponin lysis followed by extensive washes in MgCl2. Free parasites were disrupted by freezing and thawing, and the potassium content in the supernatant was determined by inductive-coupled plasma-atomic emission spectroscopy. Results are shown as percent potassium content relative to that of the control at time zero. Error bars represent the standard deviation from the mean calculated from two independent experiments performed in duplicates. If no error bar is shown, the standard deviation was smaller than the diameter of the symbol.
DISCUSSION
We have previously demonstrated that dermaseptin S4 derivatives display potent antiparasitic activity that is mediated by the lysis of the host cell. Because the antiplasmodium activity paralleled the lytic activity, albeit 30-fold-higher concentrations were needed to lyse uninfected cells, we have undertaken the development of additional derivatives with greater selectivity. We have been guided by the following rationale: selective antimicrobial activity of peptides demonstrably depends on the membrane lipid composition (19, 23, 31). The lipid compositions of the host and parasite membrane are different (37). Hence, if we can introduce the peptide into the host cell, it will be exposed to both the host cell and the parasite membranes and exert its effect on the more susceptible membrane. Increasing the lipophilicity of the peptide will render it more permeable through the host cell membrane and therefore more accessible to the parasite membrane.
This presumption has been tested experimentally by synthesizing 26 peptides with various N termini. In general, the antiplasmodium activity increased with acyl chain length up to 6 carbons and then leveled off. The impact of chain length on hemolysis displayed a minimum at C2-C4 for reasons that are not yet fully understood. We selected the most discriminating compounds based on the ratio of percent inhibition of parasite growth to percent hemolysis. For iC4-P, the ratio was 160, while for C3-P, it was 600, compared to 18 for P. It is understood that the use of this ratio for identifying the disadvantages of the most promising candidates, such as C4-Suc-P, which caused full growth inhibition at the concentration tested, and which may have also done so (while causing less hemolysis) at much lower concentrations. In other words, peptides such as C4-Suc-P might have been better candidates for further characterization than those actually chosen. Nevertheless, while such peptides will be further characterized in the future, our choice is substantiated in view of the very large difference in the ratios. These results clearly demonstrate that the acyl-peptides can affect parasite viability in a manner that is dissociated from lysis of the host cell. This suggested that the acylated peptides can (somehow) cross the host cell membrane and interact with the parasite plasma membrane to permeabilize it.
Further investigations therefore concentrated on these two acyl peptides. A detailed dose-response analysis revealed that the IC50 of the acyl peptides was ≈2.5-fold lower than that of P. In order to gain further insight into the selectivity effect, parallel determination of antiplasmodium activity and lysis of normal and infected erythrocytes were conducted for short incubation times. The IC50 values obtained in these experiments were understandably higher than those obtained in the standard dose-response test that lasts 24 h (Table 2) because of the time dependence effect. Most importantly, whereas with P, the dose dependence of growth inhibition and lysis of infected cells overlapped, for the two acyl-peptides, ≈50% growth inhibition occurred at concentrations that did not cause lysis. This discrepancy is possibly even larger, since both growth inhibition and lysis are time-dependent processes, and exposure to peptides was only 2 h in the first case and 6 h in the second. Noticeably, whereas P was more lytic to infected cells, both acylated peptides were similarly lytic to infected and uninfected erythrocytes. This is a further demonstration of lipid-dependent specificity of peptides, since the lipid composition of the membrane of infected erythrocytes is different from that of normal erythrocytes (37).
Unlike P (13), neither iC4-P nor C3-P was stage selective, being equally inhibitory for both the young ring-stage and more mature trophozoites. We interpret these results as follows: P acts essentially by lysing the host cell membrane. It is more lytic to host cells harboring mature parasite stages, indicating dependence on changes induced by the parasite in the host cell membrane. Since the latter evolves with parasite maturation, trophozoites are expected to be more sensitive than rings. In contrast, the selectivity of the acyl-peptides seems to depend on the differential composition of the host and parasite membrane. Since this is established from the onset of parasite development, the permeable acyl-peptide is always sucked into the parasite membrane and affects it. For this reason, stage dependence with acyl-peptides is neither expected nor observed.
The antiplasmodium effect was clearly time dependent. It developed gradually with time and needed the continuous presence of peptide in the parasite culture for up to 24 h in order to elicit the maximal effect. However, even the partial effect was essentially irreversible, indicating that the damage was cytotoxic. The mutual progressive aspect of the inhibition and the need for protracted exposure to the peptide may be the consequence of the gradual loss of the parasite's viability due to the membrane permeabilization. The lipophilic and membrane-trophic character of the lipopetide insinuates that its interaction with the parasite membrane would lead to its nonselective permeabilization. The observed dissipation of ΔΨ could result from increased permeability to protons, since the major generator of ΔΨ is presumably the V-type H+ pump (20, 29). Permeabilization to protons undercuts the pump's function as the major regulator of cellular pH. Short-circuiting of the pump electrogenic function presumably underlies the observed loss of cellular potassium, the maintenance of which seems to depend on ΔΨ. While these presumptions could explain the gradual and irreversible loss of vital cellular functions, at the present time, we cannot exclude the possibility that the acyl-peptides act on a different cellular target that mediates its cytotoxic action.
In conclusion, we have demonstrated in this investigation that membrane active peptides can be engineered to act specifically on the membrane of the intracellular parasite to perturb its functions. This selective activity reduces the potential harm from inadvertent lysis of the host's erythrocytes. This is a major achievement in the fine-tuning of peptide composition towards its further development as a potential antimalarial drug. It remains to be shown that the acyl-petides are not toxic to mammalian cells or to whole animals and that their antiparasitic effect is maintained in vivo. Investigations of these aspects are under way in our laboratory.
Acknowledgments
This research was supported by the Israel Science Foundation (grant no. 523/98).
The expert assistance of Josephina Silfen (Hebrew University) in peptide synthesis is gratefully acknowledged.
REFERENCES
- 1.Andreu, D., and L. Rivas. 1998. Animal antimicrobial peptides: an overview. Biopolymers 47:415-433. [DOI] [PubMed] [Google Scholar]
- 2.Blondelle, E. S., and K. Lohner. 2000. Combinatorial libraries: a tool to design antimicrobial and antifungal peptide analogues having lytic specificities for structure-activity relationship studies. Biopolymers 55:74-87. [DOI] [PubMed] [Google Scholar]
- 3.Boman, H. G. 1995. Peptide antibiotics and their role in innate immunity. Annu. Rev. Immunol. 13:61-92. [DOI] [PubMed] [Google Scholar]
- 4.Chen, J., T. J. Falla, H. Liu, M. A. Hurst, C. A. Fujii, D. A. Mosca, J. R. Embree, D. J. Loury, P. A. Radel, C. C. Chang, L. Gu, and J. C. Fiddes. 2000. Development of protegrins for the treatment and prevention of oral mucositis: structure activity relationships of synthetic protegrin analogues. Biopolymers 55:88-98. [DOI] [PubMed] [Google Scholar]
- 5.Feder, R., A. Dagan, and A. Mor. 2000. SAR study of antimicrobial dermaseptin S4 showing the consequences of peptide oligomerization on selective cytotoxicity. J. Biol. Chem. 275:4230-4238. [DOI] [PubMed] [Google Scholar]
- 6.Feder, R., R. Nechushtai, and A. Mor. 2001. Affinity driven molecular transfer from erythrocyte membrane to target cells. Peptides 22:1683-1690. [DOI] [PubMed] [Google Scholar]
- 7.Ganz, T., and R. Lehrer. 1998. Antimicrobial peptides of vertebrates. Curr. Opin. Immunol. 10:41-44. [DOI] [PubMed] [Google Scholar]
- 8.Ghosh, J. K., D. Shaool, P. Guillaud, L. Ciceron, D. Mazier, I. Kustanovich, Y. Shai, and A. Mor. 1997. Selective cytotoxicity of dermaseptin S3 toward intraerythrocytic P. falciparum and the underlying molecular basis. J. Biol. Chem. 267:6502-6509. [DOI] [PubMed] [Google Scholar]
- 9.Gura, T. 2001. Ancient system gets new respect. Science 291:2068-2071. [DOI] [PubMed] [Google Scholar]
- 10.Hancock, R. E. W., and D. S. Chapple. 1999. Peptide antibiotics. Antimicrob. Agents Chemother. 43:1317-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang, H. W. 2000. Action of antimicrobial peptides: two-state model. Biochemistry 39:8347-8352. [DOI] [PubMed] [Google Scholar]
- 12.Jaynes, J. M., C. A. Burton, S. B. Barr, G. W. Jeffers, G. R. Julian, K. L. White, F. M. Enright, T. R. Klei, and R. A. Laine. 1988. In vitro cytocidal effects of novel lytic peptides on Plasmodium falciparum and Trypanosoma cruzi. FASEB J. 2:2878-2883. [DOI] [PubMed] [Google Scholar]
- 13.Krugliak, M., R. Feder, V. Y. Zolotarev, L. Gaidukov, A. Dagan, H. Ginsburg, and A. Mor. 2000. Antiplasmodial activity of dermaseptin S4 derivatives. Antimicrob. Agents Chemother. 44:2442-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kutner, S., W. V. Breuer, H. Ginsburg, S. B. Aley, and Z. I. Cabantchik. 1985. Characterization of permeation pathways in the plasma membrane of human erythrocytes infected with early stages of Plasmodium falciparum: association with parasite development. J. Cell Physiol. 125:521-527. [DOI] [PubMed] [Google Scholar]
- 15.Lamb, H. M., and L. R. Wiseman. 1998. Pexiganan acetate. Drugs 56:1047-1054. [DOI] [PubMed] [Google Scholar]
- 16.Lambros, C. J., and J. P. Vanderberg. 1979. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 65:418-420. [PubMed] [Google Scholar]
- 17.Levy, O. 2000. Antimicrobial proteins and peptides of blood: templates for novel antimicrobial agents. Blood 96:2664-2672. [PubMed] [Google Scholar]
- 18.Maloy, L. W., and U. P. Kari. 1995. Structure-activity studies on magainins and other host defense peptides. Biopolymers 37:105-122. [DOI] [PubMed] [Google Scholar]
- 19.Martin, E., T. Ganz, and R. I. Lehrer. 1995. Defensins and other endogenous peptide antibiotics of vertebrates. J. Leukoc. Biol. 58:128-136. [DOI] [PubMed] [Google Scholar]
- 20.Mikkelsen, R. B., K. Tanabe, and D. F. Wallach. 1982. Membrane potential of Plasmodium-infected erythrocytes. J. Cell Biol. 93:685-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milik, M., and J. Skolnick. 1993. Insertion of peptide chains into lipid membranes: an off-lattice Monte Carlo dynamics model. Proteins 15:10-25. [DOI] [PubMed] [Google Scholar]
- 22.Mor, A. 2001. Peptide-based antibiotics: a potential answer to raging antimicrobial resistance. Drug Dev. Res. 50:440-447. [Google Scholar]
- 23.Mor, A., and P. Nicolas. 1994. The N-terminal alpha-helical domain 1-18 of dermaseptin is responsible for antimicrobial activity. J. Biol. Chem. 269:1934-1939. [PubMed] [Google Scholar]
- 24.Mor, A., and P. Nicolas. 1994. Isolation and structure of novel defensive peptides from frog skin. Eur. J. Biochem. 219:145-154. [DOI] [PubMed] [Google Scholar]
- 25.Mor, A., K. Hani, and P. Nicolas. 1994. The vertebrate peptide antibiotics dermaseptins have overlapping stuctural features but target specific microorganisms. J. Biol. Chem. 269:31635-31641. [PubMed] [Google Scholar]
- 26.Mor, A., V. H. Nguyen, A. Delfour, S. D. Migliore, and P. Nicolas. 1991. Isolation, amino acid sequence and synthesis of dermaseptin, a novel antimicrobial peptide of the amphibian skin. Biochemistry 3:8824-8830. [DOI] [PubMed] [Google Scholar]
- 27.Nicolas, P., and A. Mor. 1995. Peptides as weapons against microorganisms in the chemical defense system of vertebrates. Annu. Rev. Microbiol. 4:277-304. [DOI] [PubMed] [Google Scholar]
- 28.Oren, Z., and Y. Shai. 1998. Mode of action of linear amphipathic alpha-helical antimicrobial peptides. Biopolymers 47:451-463. [DOI] [PubMed] [Google Scholar]
- 29.Saliba, K. J., and K. Kirk. 1999. pH regulation in the intracellular malaria parasite P. falciparum. H+ extrusion via a V-type H+-ATPase. J. Biol. Chem. 274:33213-33219. [DOI] [PubMed] [Google Scholar]
- 30.Shai, Y. 1995. Molecular recognition between membrane-spanning polypeptides. Trends Biochem. Sci. 20:460-464. [DOI] [PubMed] [Google Scholar]
- 31.Soballe, P. W., W. L. Maloy, M. L. Myrga, L. S. Jacob, and M. Herlyn. 1995. Experimental local therapy of human melanoma with lytic magainin peptides. Int. J. Cancer 60:280-284. [DOI] [PubMed] [Google Scholar]
- 32.Sokolov, Y., T. Mirzabekov, D. W. Martin, R. I. Lehrer, and B. L. Kagan. 1999. Membrane channel formation by antimicrobial protegrins. Biochim. Biophys. Acta 1420:23-29. [DOI] [PubMed] [Google Scholar]
- 33.Strahilevitz, J., A. Mor, P. Nicolas, and Y. Shai. 1994. Spectrum of antimicrobial activity and assembly of dermaseptin b and its precursor form in phospholipid membranes. Biochemistry 33:10951-10960. [DOI] [PubMed] [Google Scholar]
- 34.Tanabe, K., A. Izumo, M. Kato, A. Miki, and S. Doi. 1989. Stage-dependent inhibiton of Plasmodium falciparum by potent Ca2+ and calmodulin modulators. J. Protozool. 36:139-143. [DOI] [PubMed] [Google Scholar]
- 35.Tossi, A., L. Sandri, and A. Giangaspero. 2000. Amphipathic, alpha-helical antimicrobial peptides. Biopolymers 55:4-30. [DOI] [PubMed] [Google Scholar]
- 36.Uematsu, N., and K. Matsuzaki. 2000. Polar angle as a determinant of amphipathic alpha-helix-lipid interactions: a model peptide study. Biophys. J. 79:2075-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vial, H. J., and M. L. Ancelin. 1998. Malarial lipids, p. 159-175. In I. W. Sherman (ed.), Malaria: parasite biology, pathogenesis, and protection. ASM Press, Washington, D.C.
- 38.Wade, D., A. Boman, B. Wahlin, C. M. Drain, D. Andreu, H. G. Boman, and R. B. Merrifield. 1990. All-D amino acid-containing channel-forming antibiotic peptides. Proc. Natl. Acad. Sci. USA 87:4761-4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White, S. H., and W. C. Wimley. 1998. Hydrophobic interactions of peptides with membrane interfaces. Biochim. Biophys. Acta 1376:339-352. [DOI] [PubMed] [Google Scholar]
- 40.World Health Organization. 1999. Disease statistics world health report. [Online.] World Health Organization, Geneva Switzerland. http://www.who.int/whr/1999/en/disease.htm.
- 41.Yang, L., T. M. Weiss, R. I. Lehrer, and H. W. Huang. 2000. Crystallization of antimicrobial pores in membranes: magainin and protegrin. Biophys. J. 79:2002-2009. [DOI] [PMC free article] [PubMed] [Google Scholar]