Abstract
We analyzed the interaction between polyamines and the outer membrane of Escherichia coli cells using potentiometric measurements with Ca2+, tetraphenylphosphonium (TPP+), and K+ electrodes. The Ca2+ electrode was used to examine the ability of the polyamines to release Ca2+ from the outer membrane. The TPP+ electrode was used to examine the ability to permeabilize the outer membrane, since the uptake of TPP+ was enhanced when the permeability barrier of the outer membrane was disrupted. The K+ electrode was used to examine permeabilization in the cytoplasmic membrane by monitoring the efflux of K+ in cytosol. Although Ca2+ release was remarkably enhanced by increasing the number of amino groups in polyamines, no TPP+ uptake was observed with polyamines of a simple structure, such as ethylenediamine, spermidine, and spermine. TPP+ uptake was observed when appropriate lipophilic moieties were further attached to the polyamines with three or four amino groups, indicating that the existence of bulky moieties as well as the number of amino groups is important to induce outer membrane permeabilization. Thus, 1-naphthylacetylspermine and N,N′-bis[6-[[(2-methoxyphenyl)methyl]amino]hexyl]-1,8-octanediamine (methoctramine) were especially effective in increasing the permeability of the outer membrane of E. coli cells, being comparable to polymyxin B nonapeptide, a well-known cationic peptide showing such action.
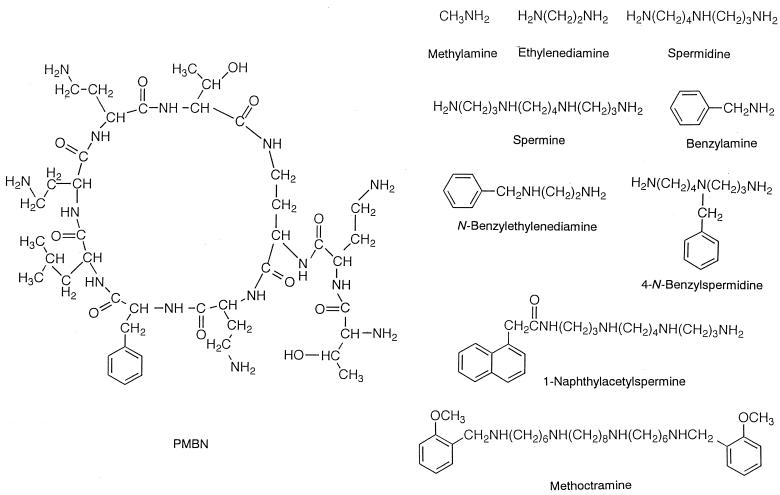
The outer membrane of gram-negative bacteria prevents the penetration of hydrophobic antibiotics into cells (5, 6, 21, 27). Many cationic compounds have been found to enhance the permeability of the outer membrane (5, 6, 21, 27). These agents, if nontoxic, could be applied to the therapy of bacterial infections to increase the permeability and sensitivity of gram-negative bacteria to various antibiotics. Polymyxin B nonapeptide (PMBN) is one of the cationic compounds (see the structure in Fig. 1) inducing outer membrane permeabilization. PMBN binds to lipopolysaccharide (LPS) molecules in the outer membrane, and this binding is considered to trigger the disruption in the permeability barrier of the membrane (6, 21, 27). Quite recently, the enantiomeric PMBN isomer, in which every amino acid residue of PMBN is replaced by its enantiomeric counterpart, was synthesized and examined for activity in the outer membrane (24). Although this peptide bound strongly to LPS, it did not permeabilize the membrane (24). Apparently, the binding of the peptide to LPS was not enough to permeabilize the outer membrane, indicating that other factors are involved. On the other hand, much simpler polyamines than PMBN have been reported to increase the permeability of the outer membrane (8, 13). These polyamines possess two or three amino groups, which were further modified with bulky hydrophobic moieties (8, 13). Thus, PMBN is not the only compound to increase the permeability of the outer membrane.
FIG. 1.
Structures of cationic compounds tested in this study. PMBN, methylamine, ethylenediamine, spermidine, spermine, benzylamine, N-benzylethylenediamine, 4-N-benzylspermidine, 1-naphthylacetylspermine, and methoctramine.
In the present study, we examined the effects of various polyamines on the outer membrane of Escherichia coli cells to clarify the basic structural requirement for permeabilization. For this purpose, we tested several cationic compounds including PMBN as shown in Fig. 1. Potentiometric measurements made with ion-selective electrodes were used, because the measurement process is fast, easy, and continuous in turbid cell suspensions (3, 7, 8, 10, 11, 13-16, 26). In this study, we used Ca2+, tetraphenylphosphonium (TPP+), and K+ electrodes. The Ca2+ electrode was used to examine the ability of the compounds to release Ca2+ from the outer membrane (14, 15). This approach seemed to be especially effective for analyzing the action of cationic compounds on the outer membrane, since these compounds are considered to expel divalent cations (such as Ca2+ and Mg2+) stabilizing LPS (14, 15). The TPP+ electrode was used to monitor the increase in permeability of the outer membrane, because the uptake of TPP+, which was stimulated by the membrane potential (inside negative) formed in the cytoplasmic membrane, was promoted when the permeability barrier of the outer membrane was disrupted (3, 8, 13-15). The K+ electrode was used to monitor the increase in permeability of the cytoplasmic membrane, which usually leads to dissipation of membrane potential, resulting in the efflux of the accumulated TPP+ (10, 16).
The final goal of the present study was to identify the fundamental structure of the polyamines inducing the outer membrane permeabilization. We found that increasing the number of amino groups and an appropriate lipophilic character are prerequisite for such action. Thus, 1-naphthylacetylspermine and methoctramine are effective in disrupting the permeability barrier of the outer membrane of E. coli cells.
MATERIALS AND METHODS
Materials.
The reagents were obtained from the following sources: calcium ionophore II (N,N,N′,N′-tetracyclohexyl-3-oxapentanediamide) and bis(2-ethylhexyl) sebacate were from Fluka (Buchs, Switzerland); potassium tetrakis(p-chlorophenyl)borate (KTpClPB), sodium tetrakis[3,5-bis(2-methoxyhexafluoro-2-propyl)phenyl]borate (NaHFPB), TPP chloride (TPPCl), EDTA, and morpholinepropanesulfonic acid (MOPS) were from Dojindo Laboratories (Kumamoto, Japan); polyvinyl chloride (PVC; degree of polymerization, 1,020) was from Nacalai Tesque (Kyoto, Japan); methoctramine tetrahydrochloride, 1-naphthylacetylspermine trihydrochloride, spermine tetrahydrochloride, spermidine trihydrochloride, benzylamine hydrochloride, 1,2-bis(2-aminophenoxy)ethane-N,N,N′N′-tetraacetic acid (BAPTA), choline chloride, and melittin (from bee venom, approximately 85% by high-performance liquid chromatography) were from Sigma (St. Louis, Mo); N-benzylethylenediamine and 4-N-benzylspermidine were from Aldrich (Milwaukee, Wis.); PMBN was from Boehringer Mannheim (Mannheim, Germany); methylamine hydrochloride and ethylenediamine dihydrochloride were from Tokyo Kasei (Tokyo, Japan); and Tris was from Wako (Osaka, Japan). All other chemicals were of analytical reagent grade.
Electrode system.
Ca2+ and TPP+ electrodes were constructed using PVC-based membranes (2, 10, 14). The PVC membranes had the following composition: Ca2+ electrode, 1 mg of calcium ionophore II, 0.6 mg of KTpClPB (50 mol% relative to the ionophore), 60 μl (about 55 mg) of bis(2-ethylhexyl) sebacate and 30 mg of PVC; TPP+ electrode, 0.5 mg of NaHFPB, 60 μl (about 55 mg) of bis(2-ethylhexyl) sebacate, and 30 mg of PVC. The materials were dissolved in tetrahydrofuran (about 1 ml) and were poured into a flat petri dish (28-mm diameter). Then the solvent was evaporated off at room temperature. The resulting membrane was excised and attached to a PVC tube (4-mm outside diameter and 3-mm inside diameter) with tetrahydrofuran adhesive. The PVC tube was filled with an internal solution comprising 10 mM CaCl2 for the Ca2+ electrode or 1 mM TPPCl and 10 mM NaCl for the TPP+ electrode, and the sensor membrane was conditioned overnight. The electrochemical cell arrangement was Ag,AgCl/internal solution/sensor membrane/sample solution/1 M NH4NO3 (salt bridge)/10 mM KCl/Ag,AgCl. A K+-selective glass electrode (type 1200) was purchased from Toko Chemical Laboratories (Tokyo, Japan). Potential measurements were made with a voltmeter produced by a field-effect transistor operational amplifier (input resistance, >1012 Ω, LF356; National Semiconductor, Sunnyvale, Calif.) connected to a recorder (LR4220E; Yokogawa, Tokyo, Japan). The detection limit was defined as the intersection of the extrapolated linear regions of the calibration graph (1). The selectivity coefficients of the electrode, ki,jPot, were determined by the fixed interference method (1) using the respective chloride salts. The constant concentrations of interfering ions were 0.5 M, except for the K+ glass electrode, which was 0.005 M for Na+ and NH4+, and the Ca2+ electrode, which was 10−6 M for TPP+. All measurements were made at room temperature (about 25°C).
Preparation of E. coli cells.
E. coli W3110, a derivative of K-12, was used. Cells were grown in minimal salt medium, supplemented with 1% Polypeptone, at 37°C under aerobic conditions (14). Cells were harvested during the exponential phase of growth, washed twice with buffer (100 mM choline chloride and 50 mM MOPS-Tris, pH 7.2), and suspended in the same buffer at 12 mg of cell protein/ml (8, 10, 12-16). The protein content was determined by the method of Lowry et al. (18).
Assay procedure.
For the measurement of Ca2+ release, the cell suspension was diluted in an assay solution containing 100 mM choline chloride, 10 mM Tris-lactate, and 50 mM MOPS-Tris (pH 7.2). The final volume was 1 ml, and the final protein concentration of E. coli cells was 0.6 mg of cell protein/ml. The Ca2+ electrode was immersed in the E. coli cell suspension, along with a miniaturized, hand-made reference electrode (10) to monitor Ca2+ release from cells. The present electrode system was compact, and, therefore, an assay solution volume as low as 1 ml could be examined. TPP+ uptake and K+ efflux were measured simultaneously. In this case, the cell suspension was diluted in an assay solution containing 100 mM choline chloride, 10 mM Tris-lactate, and 50 mM MOPS-Tris (pH 7.2), and then TPPCl was added to adjust the initial TPP+ concentration in the cell suspension to 10 μM. The final protein concentration of E. coli cells was the same as for the measurement of Ca2+, but the final volume was 2 ml. The suspension was constantly stirred with a stir-bar. Subsequently, a cationic substance dissolved in water was added and incubated for a further 3 min. In some cases, melittin was added following the addition of the cationic compound to cause further change in the permeability of the cytoplasmic membrane.
RESULTS
Response characteristics of electrodes.
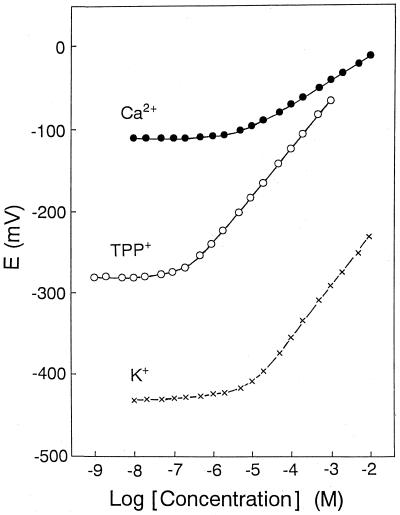
Figure 2 shows the calibration graphs of Ca2+, TPP+, and K+ electrodes. The graphs were obtained by measuring known amounts of CaCl2, TPPCl, or KCl added to a solution containing 100 mM choline chloride and 50 mM MOPS-Tris (pH 7.2) and plotting the concentrations against the obtained corresponding potential readings. All the present electrodes exhibited a near-Nernstian response. The slope and detection limit of each electrode are summarized in Table 1.
FIG. 2.
Calibration graphs for Ca2+, TPP+, and K+ electrodes in a solution containing 100 mM choline chloride and 50 mM MOPS-Tris (pH 7.2). Closed circles, Ca2+ electrode; open circles, TPP+ electrode; and crosses, K+ electrode. Electromotive force (E) between each ion-selective electrode and reference electrode is plotted against logarithmic ion concentration.
TABLE 1.
Electrode parameters
| Electrode | Slope (mV per decade) | Detection limit (μM) |
|---|---|---|
| Ca2+ electrode | 28.5 | 3.3 |
| TPP+ electrode | 58.8 | 0.2 |
| K+ electrode | 59.0 | 4.6 |
The selectivity coefficients of the electrodes are given in Table 2. The Ca2+ electrode showed no significant interference from inorganic cations, including K+, and organic cations, such as choline, used in this study. However, the interference from TPP+ was enormous. Because PVC-based membrane electrodes generally suffer significant interference from lipophilic TPP+ (9), we used a K+ glass electrode to simultaneously measure TPP+ uptake and K+ efflux. The K+ electrode showed no significant interference from TPP+ and choline. However, this electrode showed low selectivity against certain inorganic cations such as Na+ and NH4+. The TPP+ electrode showed no significant interference from either the inorganic or organic cations used in this study.
TABLE 2.
Selectivity coefficients of electrodes, log ki,jPotaa
| Interfering ion | Coefficient for:
|
||
|---|---|---|---|
| Ca2+ | TPP+ | K+ | |
| Mg2+ | −4.8 | −6.7 | −4.9 |
| Ca2+ | −6.7 | −4.5 | |
| Na+ | −4.4 | −6.5 | −1.4 |
| K+ | −4.3 | −6.3 | |
| NH4+ | −3.9 | −6.3 | −1.2 |
| Trisb | −4.8 | −6.4 | −4.8 |
| Choline | −4.7 | −6.1 | −4.9 |
| TPP+ | 5.7 | −3.7 | |
i = Ca2+ (for Ca2+ electrode), TPP+ (for TPP+ electrode), or K+ (for K+ electrode), and j = interfering ion.
pH was adjusted to 7.2 using HCl.
The response times (90% final signal) of the Ca2+, TPP+, and K+ electrodes were less than 10 s when the concentrations of Ca2+, TPP+, and K+, respectively, were changed from 50 to 100 μM in a solution containing 100 mM choline chloride and 50 mM MOPS-Tris (pH 7.2).
Action of PMBN against bacterial outer membrane as investigated with ion-selective electrodes.
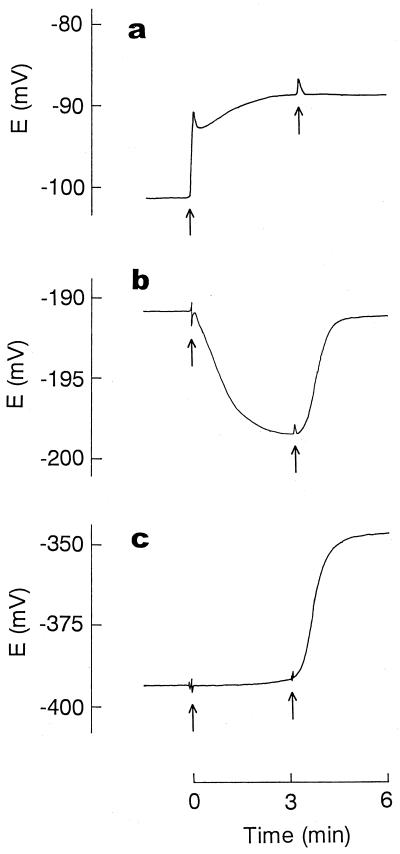
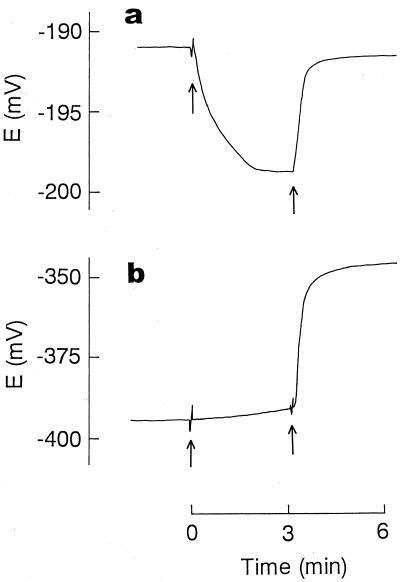
First, we used the electrodes to analyze the action of PMBN, well known as an outer membrane permeabilizer (5, 6, 21, 27), in E. coli cells. Because the Ca2+ electrode suffered serious interference from TPP+, Ca2+ release was measured independently in the absence of TPP+, while TPP+ uptake and K+ efflux were measured simultaneously. As shown in Fig. 3, Ca2+ release was observed immediately after addition of PMBN. The release implied that the structure of the outer membrane had become disorganized, since the divalent cations are essential for maintaining the LPS layer in this membrane (5, 6, 21, 27). An increase in permeability caused by the disorganization of the outer membrane structure was directly shown by the rapid uptake of TPP+ after addition of PMBN. The fact that PMBN suppressed the efflux of K+ in cytosol indicated that the peptide caused an increase in the permeability of the bacterial outer membrane only. We added a bee venom, melittin, to obtain further evidence that PMBN disrupted the permeability barrier of the outer membrane. The addition of melittin alone to cells did not enhance TPP+ uptake at all due to the barrier function of the outer membrane (6, 12, 22). When melittin was added after cells had been treated with PMBN, it caused the rapid efflux of K+ and concurrently the release of the accumulated TPP+ (Fig. 3b and c), showing that PMBN opened a pathway, through which melittin with a molecular weight of about 3,000 could pass through the outer membrane, reaching the cytoplasmic membrane to increase its K+ permeability.
FIG. 3.
Ca2+ release (a), TPP+ uptake (b), and K+ efflux (c) induced by PMBN. For the measurement of Ca2+ release (a), E. coli cells were suspended in a solution (1 ml) containing 100 mM choline chloride, 10 mM Tris-lactate, and 50 mM MOPS-Tris (pH 7.2) at 0.6 mg of cell protein/ml. TPP+ uptake (b) and K+ efflux (c) were measured simultaneously. In this case, E. coli cells were suspended at the concentration used for the Ca2+ release measurement in an assay solution (2 ml) containing 100 mM choline chloride, 10 mM Tris-lactate, 50 mM MOPS-Tris (pH 7.2), and 10 μM TPPCl. At the time indicated by the first arrow, PMBN was added at a final concentration of 100 μM. The second arrow indicates when melittin was added at a final concentration of 20 μM.
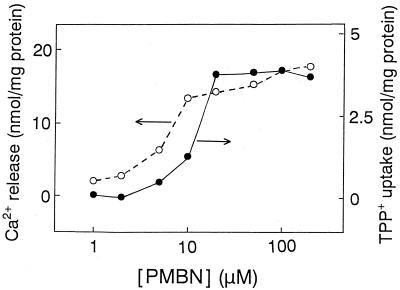
Figure 4 shows the dose-response relationship between Ca2+ release and TPP+ uptake. There was a good correlation, suggesting that PMBN stimulated TPP+ uptake immediately after the peptide induced significant Ca2+ release.
FIG. 4.
Dose-response curves for Ca2+ release and TPP+ uptake induced by PMBN.
Is the uptake of TPP+ induced whenever Ca2+ is released?
Then the question arose as to whether TPP+ uptake was always induced when Ca2+ release occurred. To answer this question, we examined the correlation between Ca2+ release and TPP+ uptake using simple cationic compounds, such as methylamine, ethylenediamine, spermidine, and spermine. For comparison, the effect of MgCl2 was also examined. The Ca2+ release and TPP+ uptake induced by the incubation of cells with 100 μM PMBN for 3 min were assigned values of 100%, and the ability of cationic substances to cause these effects was expressed as 50% effective doses (ED50s; concentrations giving 50% Ca2+ release or TPP+ uptake). These ED50s were estimated from the dose-response curves obtained by the incubation of cells with the cationic substances for 3 min. As summarized in Table 3, the concentrations required for 50% Ca2+ release lowered in the order of methylamine > ethylenediamine > spermidine > spermine, showing that the greater the number of amino groups in cationic substances, the more effective the Ca2+ release from cells. Notably, spermine with four amino groups induced Ca2+ release in the concentration range as low as that of PMBN. Nevertheless, none of the substances caused TPP+ uptake.
TABLE 3.
ED50s (concentrations giving 50% Ca2+ release and TPP+ uptake) of cationic substancesa
| Cationic substance | Concn giving:
|
|
|---|---|---|
| Ca2+ release (μM) | TPP+ uptake (μM) | |
| PMBN | 5-10 | 10-20 |
| MgCl2 | 50-100 | >500 |
| Methylamine | >500 | >500 |
| Ethylenediamine | 200-500 | >500 |
| Spermidine | 20-50 | >500 |
| Spermine | 5-10 | >500 |
Amounts of Ca2+ release and TPP+ uptake induced by the incubation of cells with 100 μM PMBN for 3 min were defined as 100%.
Then the question arose as to why these substances did not induce TPP+ uptake, though they caused Ca2+ release. It seemed likely that the substances replaced with Ca2+ can also stabilize the outer membrane. To test this possibility, we used chelating agents. We noticed that MgCl2 also elicited Ca2+ release at moderate concentrations. Thus, we expected the outer membrane replaced by Mg2+ to be safe from attack by the chelating agent BAPTA, which does not bind to Mg2+ (23), and to be attacked by EDTA having the ability to chelate with Mg2+. As control experiments, we examined the effects of two chelating agents on untreated cells. As shown in Fig. 5, both EDTA and BAPTA stimulated TPP+ uptake to eliminate the intrinsic Ca2+ stabilizing the LPS layer (Fig. 5a and b). In the Mg2+-treated cells, however, BAPTA did not increase TPP+ uptake, while EDTA did (Fig. 5c), indicating that the outer membrane replaced by Mg2+ completely escaped attack by BAPTA as was expected. Then a similar experiment was conducted using ethylenediamine-treated cells (Fig. 5d). In this case, we added EDTA, but no TPP+ uptake occurred, showing clearly that ethylenediamine also stabilizes the outer membrane. The effect of spermine was the same as that of ethylenediamine (Fig. 5e).
FIG. 5.
Effects of chelating agents on TPP+ uptake. As control experiments, EDTA (a) or BAPTA (b) was added at a final concentration of 1 mM to untreated cells at the time indicated by the arrow. (c) After E. coli cells were preincubated with 0.5 mM MgCl2 for 3 min, BAPTA and EDTA were, respectively, added at a final concentration of 1 mM at the times indicated by the first and second arrows. The cells were preincubated with 0.5 mM ethylenediamine (d) or 0.5 mM spermine (e) for 3 min, and EDTA (final concentration, 1 mM) was added at the time indicated by the arrow. EDTA and BAPTA were adjusted to pH 7.2 using Tris.
What kind of structure is required for TPP+ uptake?
To identify the structure required for uptake of TPP+, we referred to reports (8, 13) that cationic compounds with a few basic charges, which possess hydrophobic moieties, enhanced the permeability of the outer membrane. We then chose polyamines modified with an appropriate lipophilic group (such as N-benzylethylenediamine, 4-N-benzylspermidine, and 1-naphthylacetylspermine), and examined the relationship between Ca2+ release and TPP+ uptake. As in Table 3, the ability of these polyamines to cause Ca2+ release and TPP+ uptake is expressed as the ED50 (Table 4). For reference, the values of PMBN are again shown. The concentration ranges at which the polyamines with hydrophobic groups caused Ca2+ release were similar to those of the polyamines without hydrophobic groups shown in Table 3. However, we observed that 4-N-benzylspermidine and 1-naphthylacetylspermine evoked the uptake. Notably, 1-naphthylacetylspermine caused efficient TPP+ uptake in a concentration range comparable to that of PMBN. This indicates that the hydrophobic component is especially important for making TPP+ permeable, in addition to the number of amino groups. To confirm the importance of these requirements, we examined the effect of methoctramine. This compound, originally developed as an antimuscarinic agent (19), has four amino groups and two methoxyphenyl groups. It was found that methoctramine also induced TPP+ uptake at low concentrations, though the concentration range required for Ca2+ release could not be determined owing to the interference from this compound with the Ca2+ electrode. We further measured the action of melittin after adding methoctramine. As shown in Fig. 6, the addition of melittin caused a rapid efflux of K+, concomitant with the rapid release of accumulated TPP+, opening a large pathway in the outer membrane as in the case of PMBN.
TABLE 4.
ED50s (concentrations giving 50% Ca2+ release and TPP+ uptake) of polyamines with appropriate hydrophobic groupsa
| Cationic substance | Concn giving:
|
|
|---|---|---|
| Ca2+ release (μM) | TPP+ uptake (μM) | |
| PMBN | 5-10 | 10-20 |
| Benzylamine | >500 | >500 |
| N-Benzylethylenediamine | 200-500 | >500 |
| 4-N-Benzylspermidine | 20-50 | 500 |
| 1-Naphthylacetylspermine | 5-10 | 5-10 |
| Methoctramine | —b | 20-50 |
Amounts of Ca2+ release and TPP+ uptake induced by the incubation of cells with 100 μM PMBN for 3 min were defined as 100% as in Table 3.
Ca2+ release could not be measured owing to interference with the electrode.
FIG. 6.
TPP+ uptake (a) and K+ release (b) induced by methoctramine. Assay conditions were the same as for Fig. 3. At the time indicated by the first arrow, methoctramine was added at a final concentration of 100 μM. The second arrow indicates when melittin was added at a final concentration of 20 μM.
DISCUSSION
Several assay systems have been developed to analyze the interaction between cationic compounds and the bacterial outer membrane (4). The dansyl polymyxin B displacement assay and the 1-N-phenylnaphthylamine (NPN) assay using fluorescent probes are particularly effective for analyzing this interaction (4, 17, 20). Dansyl polymyxin B has been shown to bind to divalent cation-binding sites of LPS, resulting in a greatly enhanced fluorescence of the dansyl group. This property enabled us to determine the relative LPS-binding affinities of agents based on their ability to competitively displace dansyl polymyxin B from LPS. Thus, this approach is very useful for examining the binding ability of agents on LPS (4, 20). The NPN assay determines the ability of agents to permeabilize the outer membrane because, upon the destabilization of this membrane in the presence of an energy inhibitor, NPN partitions into the hydrophobic environment of the cytoplasmic membrane, where it emits a strong fluorescence (4, 17).
In this study, we made potentiometric measurements with ion-selective electrodes to analyze the actions of cationic compounds in E. coli cells. The use of ion-selective electrodes for a biochemical analysis is attractive, because the measurement is fast, easy, and continuous in a turbid cell suspension (3, 7, 8, 10, 11, 13-16, 26). Hence, ion-selective electrodes have widely been applied to the assay of membrane permeability (3, 8, 10, 13-16). For analyzing changes in the permeability of cytoplasmic membranes of living cells, the use of a K+ electrode is quite effective, because K+ is present in the cytoplasm of all kinds of cells, and the permeability-increasing action of biologically active substances in different cells can be easily compared (10, 12). Ion-selective electrodes are also useful for assaying the permeability of the bacterial outer membrane (8, 13-15). The outer membranes of gram-negative bacteria have entirely different lipid compositions from most other biological membranes, including bacterial cytoplasmic membranes (5, 6, 21, 27). The outside of the outer membrane consists of a highly charged LPS layer stabilized by divalent cations (such as Ca2+ and Mg2+), forming an effective permeability barrier against lipophilic compounds. In this case, the use of a TPP+ electrode is quite convenient (3, 8, 13-15). TPP+ cannot permeate the outer membrane, but it can pass through the cytoplasmic membrane and accumulates in the bacterial cytosol according to the membrane potential (inside negative) formed in this membrane. Hence, the uptake of TPP+ in cells occurs only when the permeability barrier of the outer membrane is disrupted (3, 8, 13-15). This process can be monitored in situ using a TPP+ electrode.
We were interested in using a Ca2+ electrode to investigate the action of biologically active substances on the outer membrane. This approach is especially effective for analyzing the action of cationic compounds on the outer membrane, since the initial function of these compounds is to expel divalent cations stabilizing LPS (8, 13). PMBN is one of the cationic compounds which can disrupt the permeability barrier of the outer membrane (5, 6, 21, 27). In this study, we found that PMBN displaced divalent cations, leading to disruption of the outer membrane, since the concentration required for Ca2+ release was almost the same as that required for TPP+ uptake. Thus, we were particularly interested in investigating the correlation between Ca2+ release and TPP+ uptake using various cationic compounds as shown in Fig. 1. As for polyamines with a simple structure, such as ethylenediamine, spermidine, and spermine, Ca2+ release but no TPP+ uptake was observed. This implied that polyamines replaced by divalent cations stabilized the outer membrane structure. In fact, this was confirmed by experiments using chelating agents; that is, EDTA did not act on cells pretreated with ethylenediamine or spermine.
We observed that polyamines with three or four amino groups were quite effective in removing Ca2+ from the outer membrane. The ED50 (5 to 10 μM) of spermine for Ca2+ release was the same as that of PMBN. To cause TPP+ uptake, it is reportedly important to have a bulky hydrophobic component (8, 13). Thus, we further modified polyamines with appropriate hydrophobic groups. As was expected, they were very effective in stimulating TPP+ uptake, indicating that the presence of a bulky hydrophobic component is quite important for making the TPP+ permeable, in addition to the number of amino groups. Notably, 1-naphthylacetylspermine and methoctramine were found to cause TPP+ uptake effectively at low concentrations, being comparable to PMBN. There was, however, a large difference in the ED50s for TPP+ uptake between 4-N-benzylspermidine (500 μM) and 1-naphthylacetylspermine (5 to 10 μM), though they have the same three amino groups and an appropriate lipophilic moiety. This indicated that the structure of the polyamines required for permeabilization of the outer membrane is very complex and that the overall structure helps to induce such action. Quite recently, Tsubery et al. (24, 25) reported that very subtle changes in the structure of PMBN greatly affected its ability to permeabilize the outer membrane, supporting the above view. Although the fundamental structure of the polyamines inducing outer membrane permeabilization has been elucidated, the structures of the most effective compounds are not entirely clear.
Acknowledgments
This work was supported by the Okayama Foundation for Science and Technology, the Wesco Science Promotion Foundation, and the Japan Society for the Promotion of Science.
REFERENCES
- 1.Buck, R. P., and E. Lindner. 1994. Recommendations for nomenclature of ion-selective electrodes. Pure Appl. Chem. 66:2527-2536. [Google Scholar]
- 2.Bühlmann, P., E. Pretsch, and E. Bakker. 1998. Carrier-based ion-selective electrodes and bulk optodes. 2. Ionophores for potentiometric and optical sensors. Chem. Rev. 98:1593-1687. [DOI] [PubMed] [Google Scholar]
- 3.Daugelavičius, R., E. Bakienė, and D. H. Bamford. 2000. Stages of polymyxin B interaction with the Escherichia coli cell envelope. Antimicrob. Agents Chemother. 44:2969-2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fidai, S., S. W. Farmer, and R. E. W. Hancock. 1997. Interaction of cationic peptides with bacterial membranes. Methods Mol. Biol. 78:187-204. [DOI] [PubMed] [Google Scholar]
- 5.Hancock, R. E. W. 1997. The bacterial outer membrane as a drug barrier. Trends Microbiol. 5:37-42. [DOI] [PubMed] [Google Scholar]
- 6.Hancock, R. E. W., T. Falla, and M. Brown. 1995. Cationic bactericidal peptides. Adv. Microb. Physiol. 37:135-175. [DOI] [PubMed] [Google Scholar]
- 7.Kamo, N., T. Racanelli, and L. Packer. 1982. Simultaneous measurements of proton movement and membrane potential changes in the wild-type and mutant Halobacterium halobium vesicles. Methods Enzymol. 88:356-360. [Google Scholar]
- 8.Katsu, T. 1991. New agents to increase the permeability of the outer membrane of Escherichia coli. Biochem. Int. 23:413-417. [PubMed] [Google Scholar]
- 9.Katsu, T. 1993. The use of tetraalkylammonium ion-sensitive electrodes for the liposome marker release assay. Anal. Chem. 65:176-180. [Google Scholar]
- 10.Katsu, T., H. Kobayashi, and Y. Fujita. 1986. Mode of action of gramicidin S on Escherichia coli membrane. Biochim. Biophys. Acta 860:608-619. [DOI] [PubMed] [Google Scholar]
- 11.Katsu, T., H. Nakagawa, T. Kanamori, N. Kamo, and T. Tsuchiya. 2001. Ion-selective electrode for transmembrane pH difference measurements. Anal. Chem. 73:1849-1854. [DOI] [PubMed] [Google Scholar]
- 12.Katsu, T., M. Kuroko, T. Morikawa, K. Sanchika, Y., Fujita, H. Yamamura, and M. Uda. 1989. Mechanism of membrane damage induced by the amphipathic peptides gramicidin S and melittin. Biochim. Biophys. Acta 983:135-141. [DOI] [PubMed] [Google Scholar]
- 13.Katsu, T., M. Shibata, and Y. Fujita. 1985. Dication and trication which can increase the permeability of Escherichia coli outer membrane. Biochim. Biophys. Acta 818:61-66. [DOI] [PubMed] [Google Scholar]
- 14.Katsu, T., S. Yoshimura, T. Tsuchiya, and Y. Fujita. 1984. Temperature dependence of action of polymyxin B on Escherichia coli. J. Biochem. 95:1645-1653. [DOI] [PubMed] [Google Scholar]
- 15.Katsu, T., S. Yoshimura, and Y. Fujita. 1984. Increases in permeability of Escherichia coli outer membrane induced by polycations. FEBS Lett. 166:175-178. [DOI] [PubMed] [Google Scholar]
- 16.Katsu, T., T. Tsuchiya, and Y. Fujita. 1984. Dissipation of membrane potential of Escherichia coli cells induced by macromolecular polylysine. Biochem. Biophys. Res. Commun. 122:401-406. [DOI] [PubMed] [Google Scholar]
- 17.Loh, B., C. Grant, and R. E. W. Hancock. 1984. Use of the fluorescent probe 1-N-phenylnaphthylamine to study the interactions of aminoglycoside antibiotics with the outer membrane of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 26:546-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 19.Melchiorre, C. 1990. Polymethylene tetraamines: a novel class of cardioselective M2-antagonists. Med. Res. Rev. 10:327-349. [DOI] [PubMed] [Google Scholar]
- 20.Moore, R. A., N. C. Bates, and R. E. W. Hancock. 1986. Interaction of polycationic antibiotics with Pseudomonas aeruginosa lipopolysaccharide and lipid A studied by using dansyl-polymyxin. Antimicrob. Agents Chemother. 29:496-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nikaido, H. 1996. Outer membrane, p. 29-47. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 22.Piers, K. L., M. H. Brown, and R. E. W. Hancock. 1994. Improvement of outer membrane-permeabilizing and lipopolysaccharide-binding activities of an antimicrobial cationic peptide by C-terminal modification. Antimicrob. Agents Chemother. 38:2311-2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsien, R. Y. 1980. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry 19:2396-2404. [DOI] [PubMed] [Google Scholar]
- 24.Tsubery, H., I. Ofek, S. Cohen, and M. Fridkin. 2000. The functional association of polymyxin B with bacterial lipopolysaccharide is stereospecific: studies on polymyxin B nonapeptide. Biochemistry 39:11837-11844. [DOI] [PubMed] [Google Scholar]
- 25.Tsubery, H., I. Ofek, S. Cohen, and M. Fridkin. 2000. Structure-function studies of polymyxin B nonapeptide: implications to sensitization of Gram-negative bacteria. J. Med. Chem. 43:3085-3092. [DOI] [PubMed] [Google Scholar]
- 26.Tsuchiya, T., and T. H. Wilson. 1978. Cation-sugar cotransport in the melibiose transport system of Escherichia coli. Membr. Biochem. 2:63-79. [DOI] [PubMed] [Google Scholar]
- 27.Vaara, M. 1992. Agents that increase the permeability of the outer membrane. Microbiol. Rev. 56:395-411. [DOI] [PMC free article] [PubMed] [Google Scholar]