Abstract
The molecular controls regulating the successful colonization of Candida albicans on foreign materials are not known. Here we show that a mutant C. albicans strain defective in filamentous growth and lacking the transcription factors Efg1p and Cph1p has a profoundly deficient potential for colonizing on polyurethane catheters.
The central venous catheter is known to be a major reservoir and source for Candida bloodstream infections in hospitalized patients (5). In addition, the ability of Candida species to adhere to foreign materials such as catheters has been well demonstrated (7). However, the molecular controls and environmental cues that regulate successful colonization of Candida spp. on such materials are not fully known. A number of pathogenicity and virulence factors that may contribute to successful colonization of Candida albicans have been proposed; of these, the greatest interest has been paid to the ability of Candida spp. to switch from yeast to filamentous growth. Dimorphism in C. albicans is regulated by at least two pathways, a conserved mitogen-activated protein kinase pathway that modulates the transcription factor Cph1p and a cyclic AMP-protein kinase A pathway that regulates the transcription factor Efg1p (2). A mutant C. albicans strain lacking both the transcription factors Efg1p (9) and Cph1p (3) is profoundly defective in filamentous growth (4), even though an Efg1p- and Cph1p-independent pathway for filamentous growth exists (6). We examined the abilities of the efg1/efg1 cph1/cph1 double-null mutant and the efg1/efg1 cph1/cph1 double mutant with the wild-type EFG1 gene in the genome, as well as those of the cph1/cph1 and efg1/efg1 single mutants and isogenic wild-type C. albicans strains, to colonize on foreign materials (polyurethane catheters).
The strains used in this study and their genotypes were as follows: CAI4, ura3::1 imm434/ura3::1 imm434; JKC18, ura3::1 im434/ura3::1 imm434 cph1::hisG/cph1::hisG-URA3-hisG; HLC67, ura3::1 imm434/ura3::1 imm434 efg1::hisG/efg1::hisG-URA3-hisG; HLC69, ura3::1 imm434/ura3::1 imm434 cph1::hisG/cph1::hisG efg1::hisG/efg1::hisG-URA3-hisG; andHLC84, ura3::1 imm434/ura3::1 imm434 cph1::hisG/cph1::hisG efg1::hisG/efg1::hisG (EFG1-URA3) (see reference 1 for strain CAI4, reference 3 for strain JKC18, and reference 4 for the remaining strains). Human plasma (10 ml) was injected through the lumen ports of single-lumen 7-French polyurethane catheters (Cook Catheters, Bloomington, Ind.), which were then incubated at 35°C for 24 h. Next, the catheters were removed from the plasma and placed in a culture medium (RPMI 1640 plus 0.165 M MOPS [morpholinepropanesulfonic acid]; pH 7.0) containing standardized suspensions (105 CFU/ml) of the C. albicans strains tested that were prepared from a 24-h culture plate. After incubation (35°C) in this medium for an additional 24 h, the catheters were carefully removed and flushed via the lumen with 100 ml of sterile saline. The catheters were then placed in a separate sterile culture tube containing 10 ml of sterile saline and gently vortexed to remove nonadherent organisms from outside the catheter lumen. The catheters were subsequently removed from the saline, aseptically cut at the 6-cm mark (on the catheter lumen), and placed in 10 ml of fresh sterile culture medium. The culture tubes were then sonicated (55,000 Hz) for 5 min to remove adherent organisms (8). After an additional 15 s of vortexing, a 50-μl sample of the culture medium was removed to a potato dextrose agar (Remel, Lenexa, Kans.) plate using a spiral-gradient plating system (Spiral Biotech Inc., Bethesda, Md.). The numbers of viable CFU per milliliter of medium were determined according to the colony counts after 24- and 48-h incubations at 35°C. To further characterize the phenotype of catheter attachments by the wild-type and mutant C. albicans strains, experiments were repeated as described above except that the 6-cm catheter segment was placed in 4% glutaraldehyde in a 0.1 M sodium cacodylate buffer, pH 7.2. Catheter sections (2 cm) were then fixed in osmium tetroxide and dehydrated using a graded series of ethanol washings followed by immersion in hexamethyldisilazane. Next, the sections were air dried, mounted onto aluminum stubs, and sputter-coated with Pd and Au. A Hitachi S4000 scanning electron microscope (Hitachi Scientific Instruments, Mountain View, Calif.) was used for visualization of the samples. All C. albicans strains were tested simultaneously, and experiments were performed in triplicate. Mean data (CFU per milliliter)were compared using analysis of variance along with the Tukey's test for post hoc comparisons.
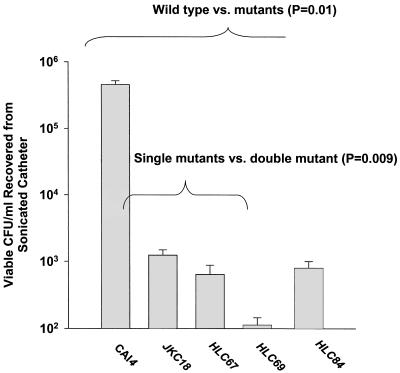
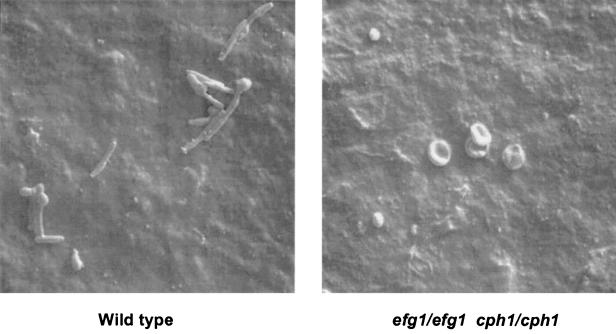
The efg1/efg1 cph1/cph1 double-mutant strain was defective in its ability to colonize on the polyurethane catheters. Specifically, the mean number of viable CFU per milliliter recovered from the sonicated catheter segments was 3 to 4 log10 lower for the double-mutant strain versus the wild-type strain (P = 0.01) (Fig. 1). The efg1/efg1 and cph1/cph1 single-mutant strains had an intermediate phenotype, having a mean number of viable CFU per milliliter recovered from the segments that was about 1 log10 higher than that for the double-mutant strain (Fig. 1). When catheters were examined by scanning electron microscopy, decreased colonization was noted by the efg1/efg1 cph1/cph1 double mutant, with occasional blastoconidia but no hyphal forms (Fig. 2).
FIG. 1.
Comparison of the colonization intensities (polyurethane central venous catheters) of C. albicans strains with the following genotypes: isogenic wild-type (CAI4), efg1/efg1 (HLC67) and cph1/cph1 (JKC18) single mutants, efg1/efg1 cph1/cph1 double mutant (HLC69), and efg1/efg1 cph1/cph1 with the wild-type EFG1 gene (HLC84). Counts were analyzed by nonparametric Kruskal-Wallis one-way analysis of variance on ranks by using Dunn's pairwise multiple-comparison technique (Sigmastat statistical software, version 2.0; SPSS Science, Chicago, Ill.).
FIG. 2.
Scanning electron micrographs (magnification, ×1,000) of exterior catheter lumens exposed to the wild-type (CAI4) and efg1/efg1 cph1/cph1 double-mutant (HLC69) C. albicans strains.
Of the two major signaling pathways controlling hyphal growth, the Efg1p pathway appears more important since egf1/efg1 mutants demonstrate a more severe morphogenetic block under most inducing conditions (4). Furthermore, egf1/efg1 mutants are much less virulent than the cph1/cph1 mutants in the mouse model of candidiasis (4). Our observation that the efg1/efg1 mutant had more-severe adherence defects than the cph1/cph1 mutant is consistent with these prior observations. Nevertheless, the efg1/efg1 cph1/cph1 double mutant has the most severe morphogenetic and virulence defects (4). Such morphogenetic defects were also observed in our study. The presence of the wild-type EFG1 gene integrated in the genome of the efg1/efg1 cph1/cph1 double mutant (HLC84) caused the formation of germ tubes and pseudohyphae (4). The ability of the efg1/efg1 cph1/cph1 double mutant to colonize was restored to the level of the cph1/cph1 single mutant by integrating the wild-type EFG1 gene in the genome (Fig. 1). Therefore, our observation is consistent with the hypothesis that Cph1p and Efg1p govern catheter colonization ability.
One of the limitations of our approach is that we did not evaluate fully the possibility of another, secondary mutation giving the phenotype observed. However, we think that it is unlikely that another mutation could affect the phenotypic characteristics in two genotypically different strains such as efg1/efg1 and cph1/cph1. In addition, our preliminary data do not address which of the rather-complex and interrelated early events of catheter colonization, such as adherence and biofilm formation, are regulated predominantly by EFG1 and CPH1.
In conclusion, both Efg1p and Cph1p appear to be central regulators of the developmental program that allows C. albicans to successfully colonize on foreign materials such as polyurethane central venous catheters. It is possible that interruption of morphogenetic events induced by Efg1p and Cph1p through pharmacological or other means could affect the fitness of C. albicans to successfully colonize prosthetic materials or central venous catheters.
REFERENCES
- 1.Fonzi, W. A., and M. Y. Irwin, 1993. Isogenic strain construction and gene mapping in Candida albicans. Genetics 134:717-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lengeler, K. B., R. C. Davidson, C. D'Sousa, et al. 2000. Signal transduction regulating fungal development and virulence. Microbiol. Mol. Biol. Rev. 64:746-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu, H., J. Kohler, and G. R. Fink. 1994. Suppression of hyphal formation in Candida albicans by mutation of a STE12 homolog. Science 266:1723-1726. [DOI] [PubMed] [Google Scholar]
- 4.Lo, H. J., J. R. Kohler, B. DiDomenico, D. Loebenberg, A. Cacciapuoti, and G. R. Fink. 1997. Nonfilamentous C. albicans mutants are avirulent. Cell 90:939-949. [DOI] [PubMed] [Google Scholar]
- 5.Pfaller, M. A. 1996. Nosocomial candidiasis: emerging species, reservoirs, and modes of transmission. Clin. Infect. Dis. 22(Suppl. 2):S89-S94. [DOI] [PubMed]
- 6.Riggle, P. J., K. A. Andrutis, X. Chen, S. R. Tzipori, and C. A. Kumamoto. 1999. Invasive lesions containing filamentous forms produced by a Candida albicans mutant that is defective in filamentous growth in culture. Infect. Immun. 67:3649-3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rotrosen, D., T. R. Gibson, and J. E. Edwards, Jr. 1983. Adherence of Candida species to intravenous catheters. J. Infect. Dis. 147:594.. [DOI] [PubMed] [Google Scholar]
- 8.Sherertz, R. J., I. I. Raad, A. Belani, L. C. Koo, K. H. Rand, D. L. Pickett, S. A. Straub, and L. L. Fauerbach. 1990. Three-year experience with sonicated vascular catheter cultures in a clinical microbiology laboratory. J. Clin. Microbiol. 28:76-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stoldt, V. R., A. Sonneborn, C. E. Leuker, and J. F. Ernst. 1997. Efg1p, an essential regulator of morphogenesis of the human pathogen Candida albicans, is a member of a conserved class of bHLH proteins regulating morphogenetic processes in fungi. EMBO J. 16:1982-1991. [DOI] [PMC free article] [PubMed] [Google Scholar]