Abstract
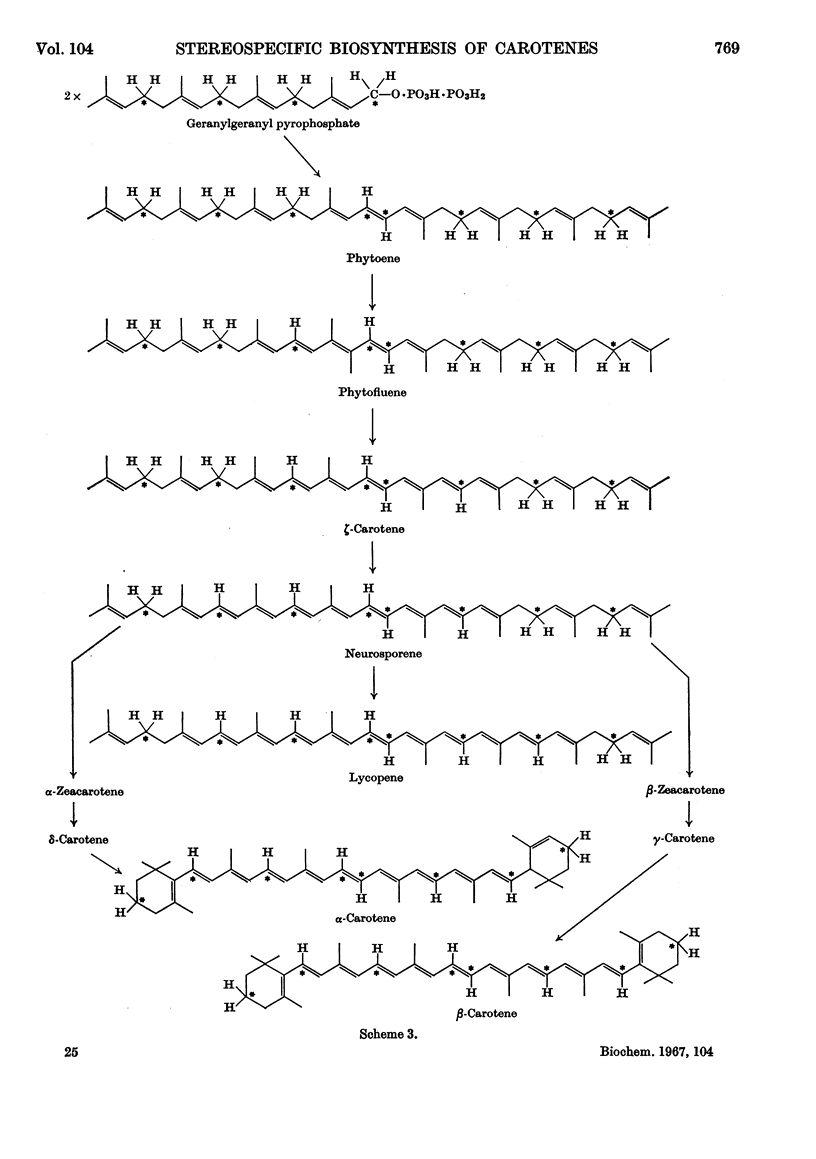
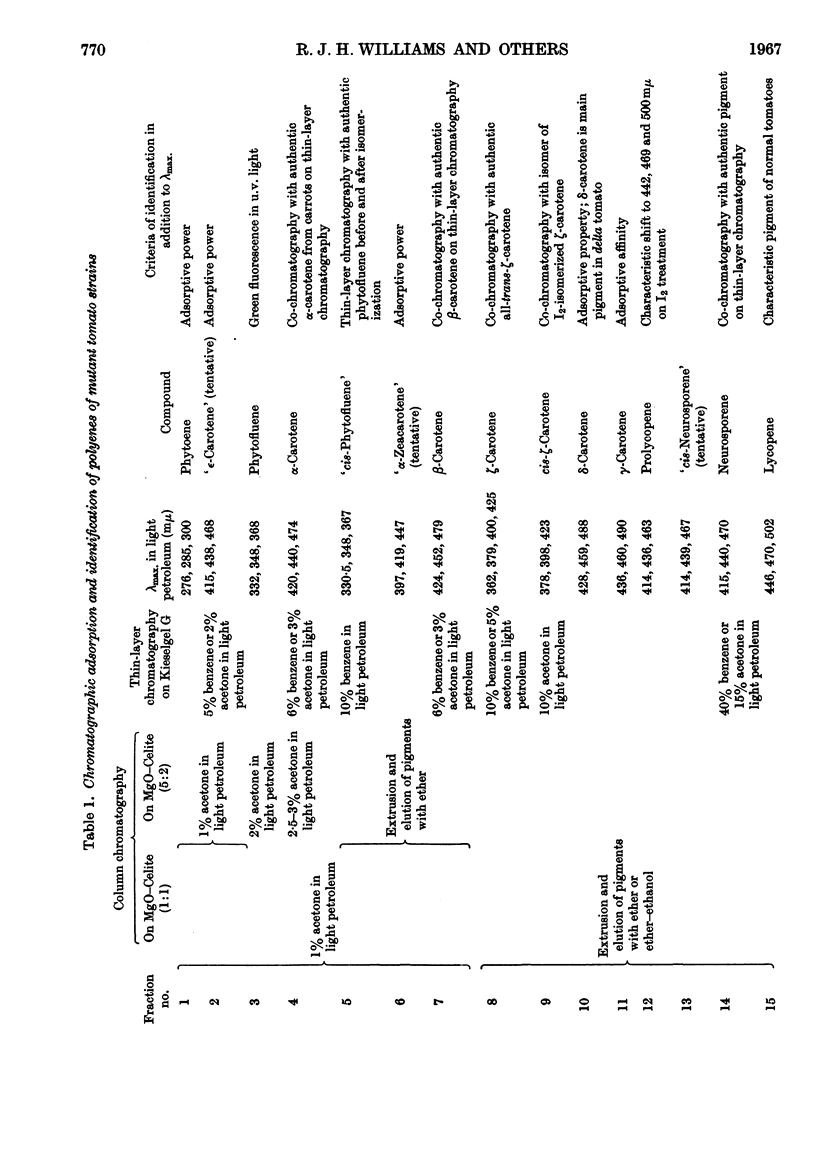
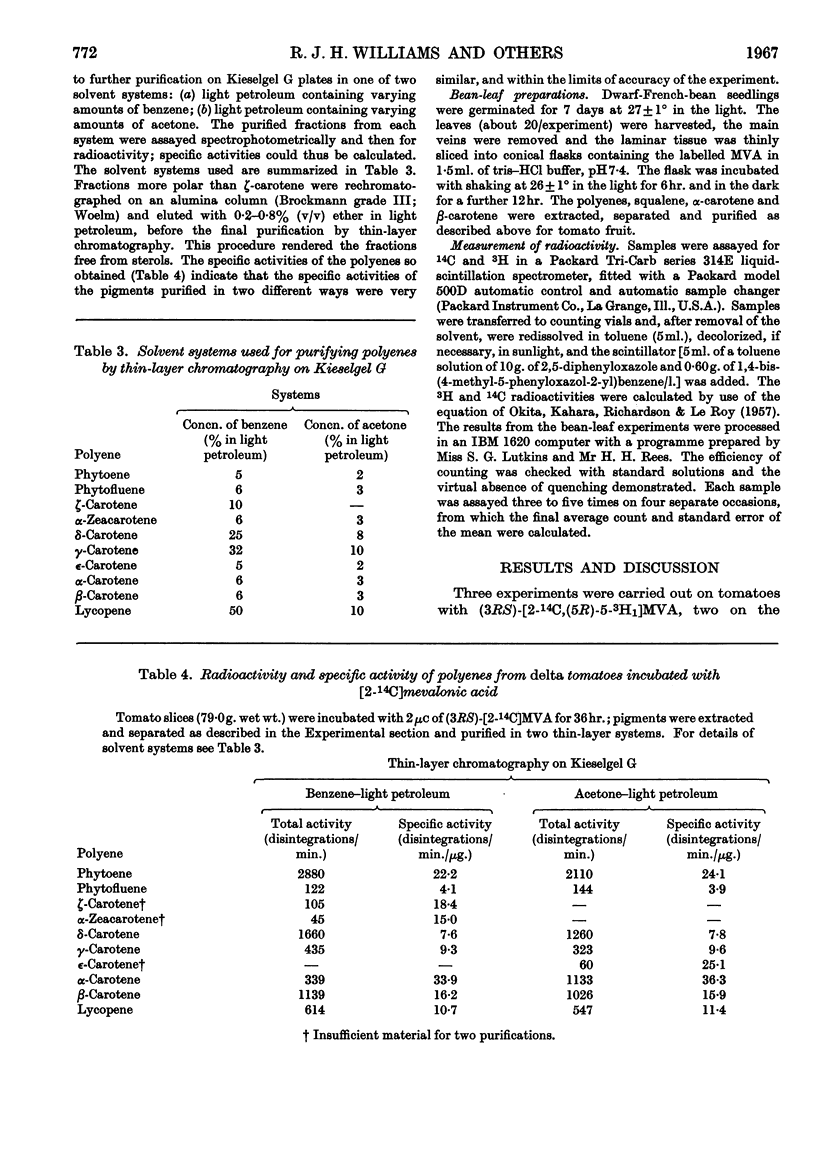
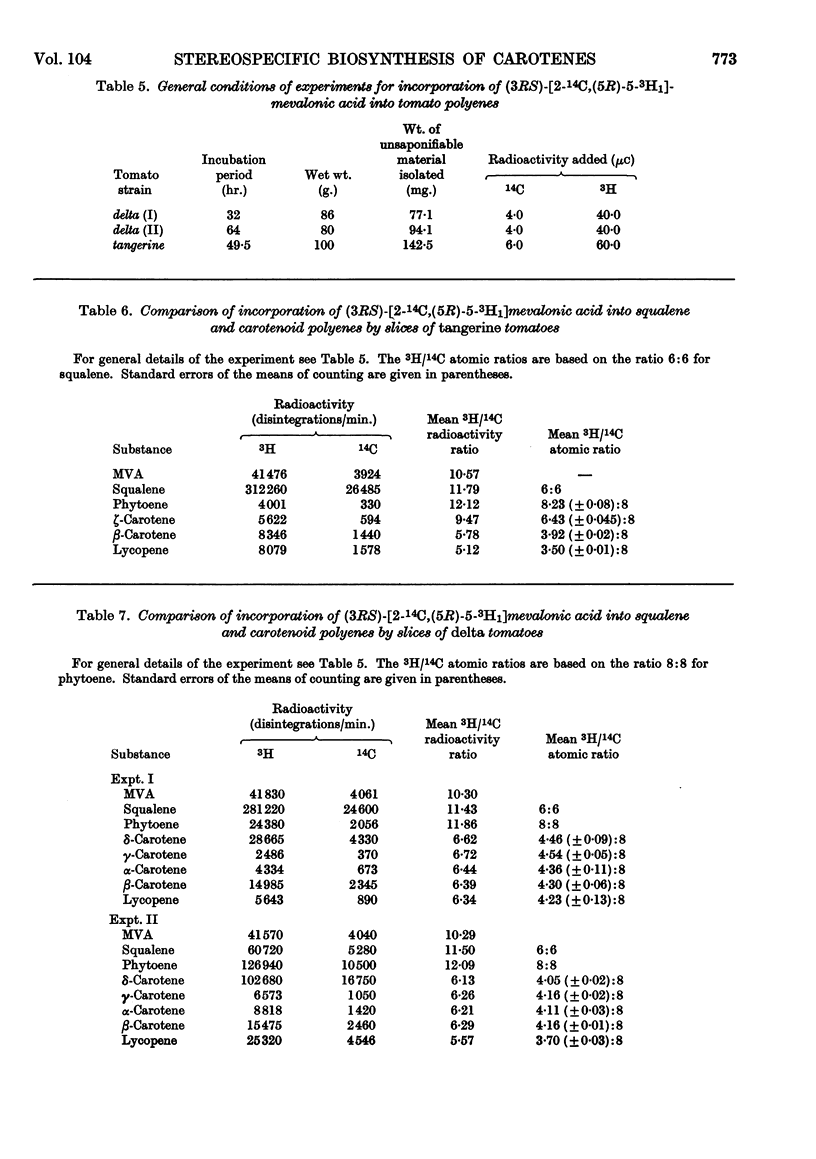
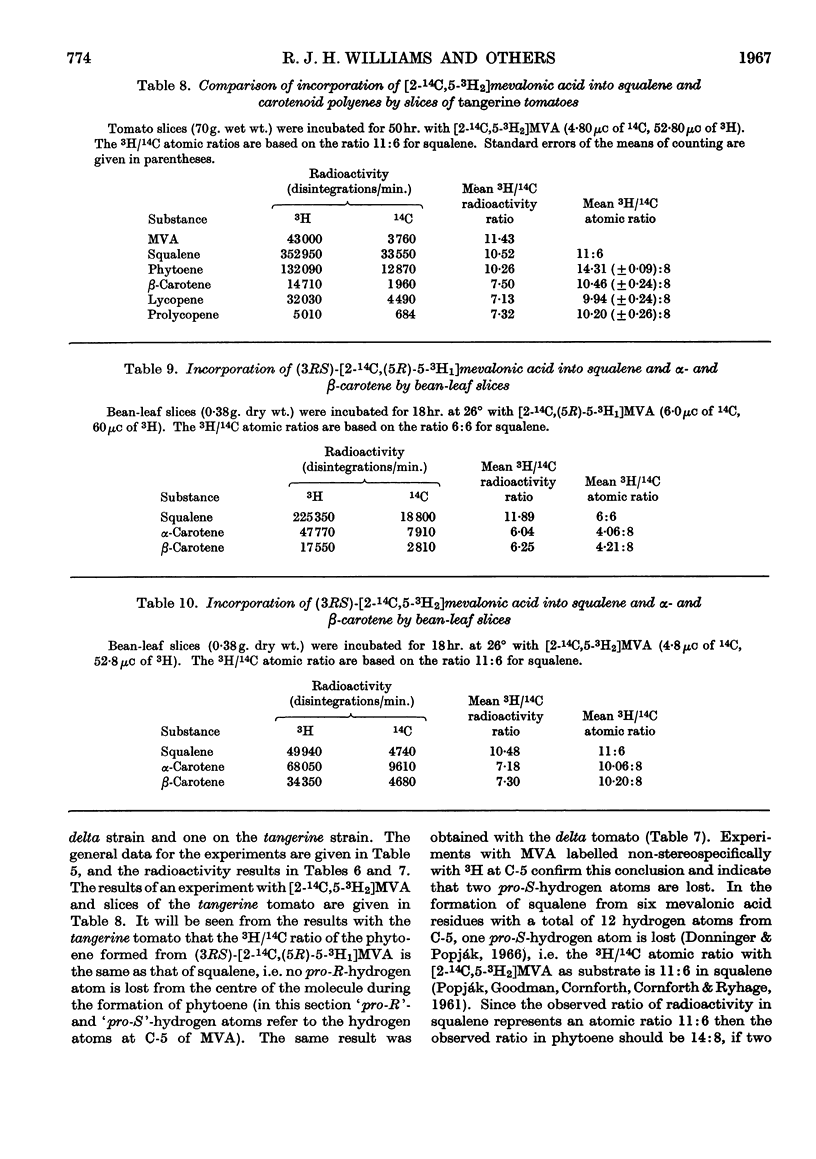
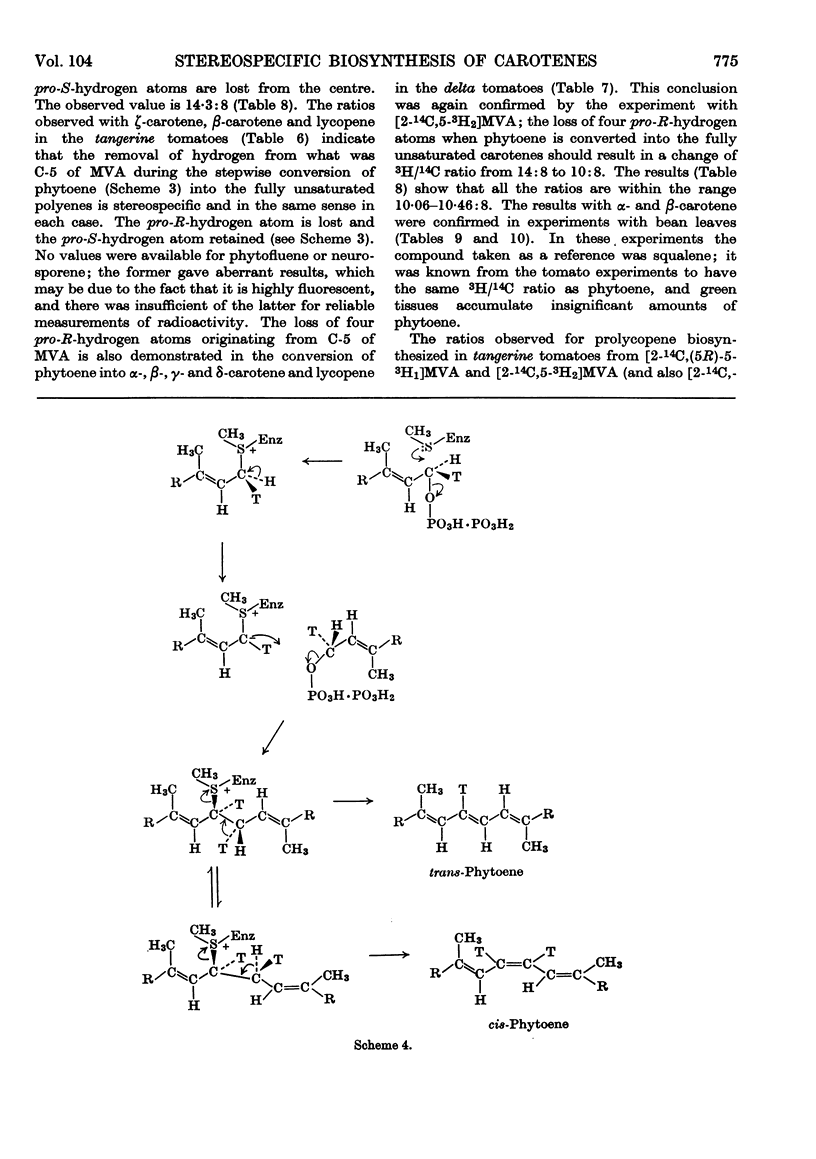
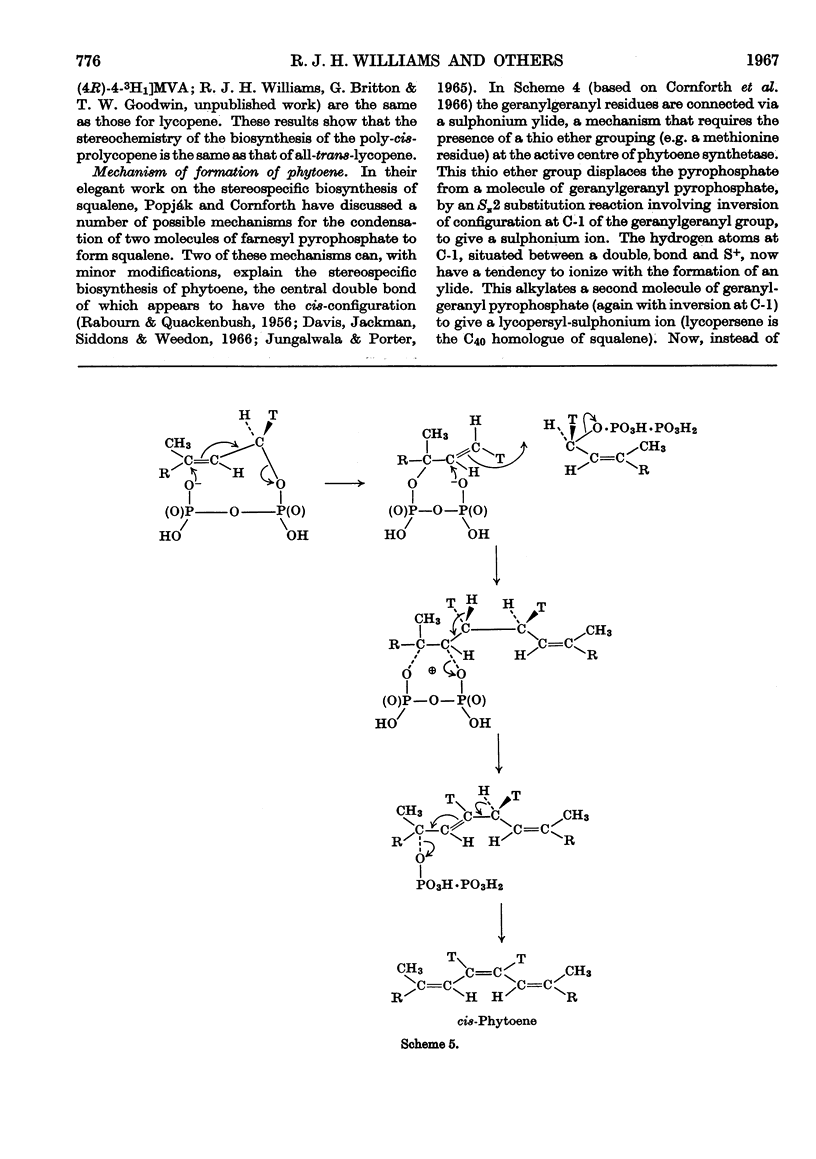
1. The incorporation of [2-14C,(5R)-5-3H1]mevalonic acid and [2-14C,5-3H2]-mevalonic acid into phytoene, phytofluene, ζ-carotene, neurosporene, α-, β-, γ- and δ-carotene and lycopene by slices of fruit from two tomato mutants (delta and tangerine) and into α- and β-carotene by bean leaves has been studied. 2. In the formation of phytoene, all the pro-R-hydrogen atoms from C-5 of mevalonic acid are retained whereas two pro-S-hydrogen atoms are lost. 3. Possible mechanisms for the condensation of two molecules of all-trans-geranylgeranyl pyrophosphate are outlined. 4. In each dehydrogenation step from phytoene to the fully unsaturated carotenes, one pro-R-hydrogen atom from C-5 of mevalonic acid is lost, indicating that the sequential dehydrogenation is stereospecific and in the same sense at each step.
Full text
PDF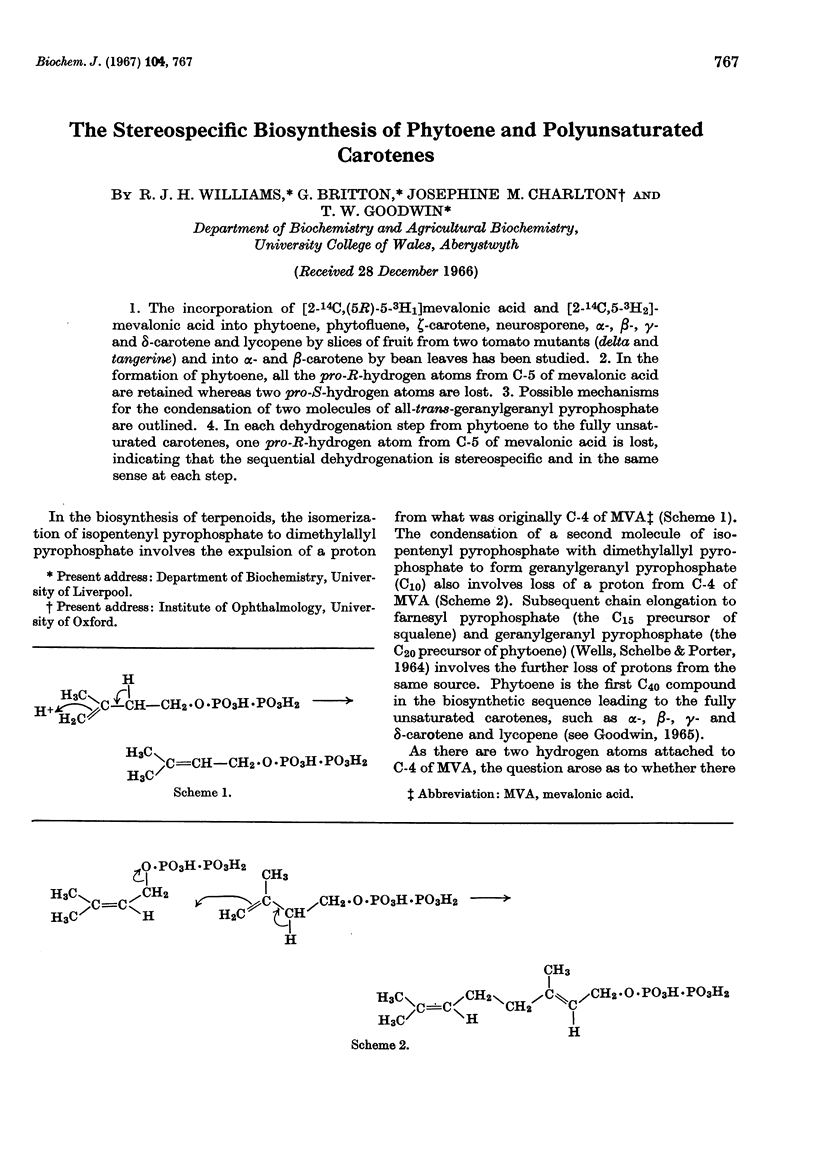



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CORNFORTH J. W., CORNFORTH R. H., POPJAK G., GORE I. Y. Studies on the biosynthesis of cholesterol. 5. Biosynthesis of squalene from DL-3-hydroxy-3-methyl [2-14C] pentano-5-lactone. Biochem J. 1958 May;69(1):146–155. doi: 10.1042/bj0690146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORNFORTH J. W., HUNTER G. D., POPJAK G. Studies of cholesterol biosynthesis. II. Distribution of acetate carbon in the ring structure. Biochem J. 1953 Jul;54(4):597–601. doi: 10.1042/bj0540597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaykin S., Meissner L. The borohydride reduction products of DPN. Biochem Biophys Res Commun. 1964;14:233–240. doi: 10.1016/0006-291x(64)90441-3. [DOI] [PubMed] [Google Scholar]
- Cornforth J. W., Cornforth R. H., Donninger C., Popják G. Studies on the biosynthesis of cholesterol XIX. Steric course of hydrogen eliminations and of C-C bond formations in squalene biosynthesis. Proc R Soc Lond B Biol Sci. 1966 Jan 18;163(993):492–514. doi: 10.1098/rspb.1966.0004. [DOI] [PubMed] [Google Scholar]
- Donninger C., Popják G. Studies on the biosynthesis of cholesterol. 18. The stereospecificity of mevaldate reductase and the biosynthesis of asymmetrically labelled farnesyl pyrophosphate. Proc R Soc Lond B Biol Sci. 1966 Jan 18;163(993):465–491. doi: 10.1098/rspb.1966.0003. [DOI] [PubMed] [Google Scholar]
- JUNGALWALA F. B., PORTER J. W. THE CONFIGURATION OF PHYTOENE. Arch Biochem Biophys. 1965 May;110:291–299. doi: 10.1016/0003-9861(65)90121-9. [DOI] [PubMed] [Google Scholar]
- Krishna G., Whitlock H. W., Jr, Feldbruegge D. H., Porter J. W. Enzymic conversion of farnesyl pyrophosphate to squalene. Arch Biochem Biophys. 1966 Apr;114(1):200–215. doi: 10.1016/0003-9861(66)90322-5. [DOI] [PubMed] [Google Scholar]
- POPJAK G., GOODMAN W. S., CORNFORTH J. W., CORNFORTH R. H., RYHAGE R. Studies on the biosynthesis of cholesterol. XV. Mechanism of squalene biosynthesis from farnesyl pyrophosphate and from mevalonate. J Biol Chem. 1961 Jul;236:1934–1947. [PubMed] [Google Scholar]
- Popják G., Cornforth J. W. Substrate stereochemistry in squalene biosynthesis: The first Ciba medal lecture. Biochem J. 1966 Dec;101(3):553.b4–553568. [PMC free article] [PubMed] [Google Scholar]
- RABOURN W. J., QUACKENBUSH F. W. The structure of phytoene. Arch Biochem Biophys. 1956 Mar;61(1):111–118. doi: 10.1016/0003-9861(56)90321-6. [DOI] [PubMed] [Google Scholar]
- Rilling H. C. A new intermediate in the biosynthesis of squalene. J Biol Chem. 1966 Jul 10;241(13):3233–3236. [PubMed] [Google Scholar]
- SCHLESINGER M. J., COON M. J. Reduction of mevaldic acid to mevalonic acid by a partially purified enzyme from liver. J Biol Chem. 1961 Sep;236:2421–2424. [PubMed] [Google Scholar]
- Williams R. J., Britton G., Goodwin T. W. A possible mechanism for the biosynthesis of eschscholtzxanthin. Biochim Biophys Acta. 1966 Jul 27;124(1):200–203. doi: 10.1016/0304-4165(66)90333-3. [DOI] [PubMed] [Google Scholar]


