Abstract
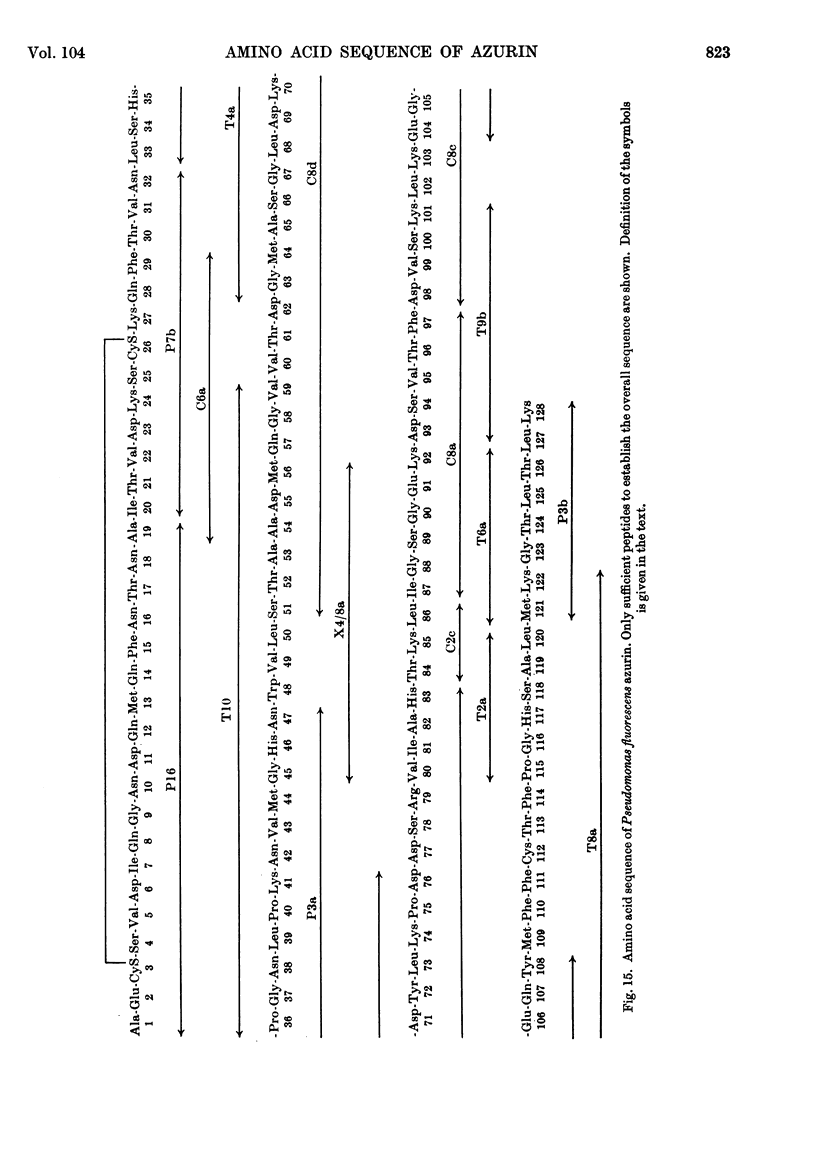
The amino acid sequence of Pseudomonas fluorescens azurin has been determined. The protein consists of a single peptide chain of 128 residues. There is one intra-chain disulphide bridge. The sequence was determined by isolation of the soluble tryptic peptides, and by exhaustive examination of the products of chymotryptic and peptic digestion. The sequence has been confirmed by the purification and analysis of the seven fragments obtained by cyanogen bromide treatment of the protein.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACHER R., FROMAGEOT C., JUTISZ M. Séparations chromatographiques d'acides aminés et de peptides; les acides aminés et de peptides; les acides aminés individuels de l'insuline avec une note sur le dosage de la proline. Biochim Biophys Acta. 1950 Mar;5(1):81–88. doi: 10.1016/0006-3002(50)90151-x. [DOI] [PubMed] [Google Scholar]
- AMBLER R. P., BROWN L. H. THE AMINO ACID SEQUENCE OF PSEUDOMONAS FLUORESCENS AZURIN. J Mol Biol. 1964 Sep;9:825–828. doi: 10.1016/s0022-2836(64)80188-1. [DOI] [PubMed] [Google Scholar]
- AMBLER R. P. THE AMINO ACID SEQUENCE OF PSEUDOMONAS CYTOCHROME C-551. Biochem J. 1963 Nov;89:349–378. doi: 10.1042/bj0890349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMBLER R. P. THE PURIFICATION AND AMINO ACID COMPOSITION OF PSEUDOMONAS CYTOCHROME C-551. Biochem J. 1963 Nov;89:341–349. doi: 10.1042/bj0890341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROMAN L., MALMSTROEM B. G., AASA R. THE ROLE OF COPPER IN THE CATALYTIC ACTION OF LACCASE AND CERULOPLASMIN. Biochim Biophys Acta. 1963 Nov 29;75:365–376. doi: 10.1016/0006-3002(63)90624-3. [DOI] [PubMed] [Google Scholar]
- Brown J. R., Hartley B. S. Location of disulphide bridges by diagonal paper electrophoresis. The disulphide bridges of bovine chymotrypsinogen A. Biochem J. 1966 Oct;101(1):214–228. doi: 10.1042/bj1010214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COVAL M. L., HORIO T., KAMEN M. D. The amino acid compositions of some bacterial haem proteins. Biochim Biophys Acta. 1961 Aug 5;51:246–259. doi: 10.1016/0006-3002(61)90165-2. [DOI] [PubMed] [Google Scholar]
- GRAY W. R., HARTLEY B. S. THE STRUCTURE OF A CHYMOTRYPTIC PEPTIDE FROM PSEUDOMONAS CYTOCHROME C-551. Biochem J. 1963 Nov;89:379–380. doi: 10.1042/bj0890379. [DOI] [PubMed] [Google Scholar]
- HIRS C. H. The oxidation of ribonuclease with performic acid. J Biol Chem. 1956 Apr;219(2):611–621. [PubMed] [Google Scholar]
- Haley E. E., Corcoran B. J., Dorer F. E., Buchanan D. L. Beta-aspartyl peptides in enzymatic hydrolysates of protein. Biochemistry. 1966 Oct;5(10):3229–3235. doi: 10.1021/bi00874a024. [DOI] [PubMed] [Google Scholar]
- KONIGSBERG W., HILL R. J. The structure of human hemoglobin. III. The sequence of amino acids in the tryptic peptides of the alpha chain. J Biol Chem. 1962 Aug;237:2547–2561. [PubMed] [Google Scholar]
- Milstein C. The disulphide bridges of immunoglobulin kappa-chains. Biochem J. 1966 Nov;101(2):338–351. doi: 10.1042/bj1010338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKAMURA T. Purification and physico-chemical properties of laccase. Biochim Biophys Acta. 1958 Oct;30(1):44–52. doi: 10.1016/0006-3002(58)90239-7. [DOI] [PubMed] [Google Scholar]
- Offord R. E. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature. 1966 Aug 6;211(5049):591–593. doi: 10.1038/211591a0. [DOI] [PubMed] [Google Scholar]
- SMITHIES O. Zone electrophoresis in starch gels and its application to studies of serum proteins. Adv Protein Chem. 1959;14:65–113. doi: 10.1016/s0065-3233(08)60609-9. [DOI] [PubMed] [Google Scholar]
- SUTHERLAND I. W., WILKINSON J. F. Azurin: a copper protein found in Bordetella. J Gen Microbiol. 1963 Jan;30:105–112. doi: 10.1099/00221287-30-1-105. [DOI] [PubMed] [Google Scholar]
- SUZUKI H., IWASAKI H. Studies on denitrification. VI. Preparations and properties of crystalline blue protein and cryptocytochrome c, and role of copper in denitrifying enzyme from a denitrifying bacterium. J Biochem. 1962 Sep;52:193–199. doi: 10.1093/oxfordjournals.jbchem.a127596. [DOI] [PubMed] [Google Scholar]
- YAMANAKA T., KIJIMOTO S., OKUNUKI K. Some properties of Pseudomonas blue protein and its apo-protein. J Biochem. 1963 Mar;53:256–259. doi: 10.1093/oxfordjournals.jbchem.a127689. [DOI] [PubMed] [Google Scholar]


