Abstract
The interactions of artemisinin with atovaquone, quinine, and mefloquine were investigated in three Plasmodium falciparum strains (strains F-32, FCR-3, and K-1) by an in vitro culture assay. The parasites were cultured for 48 h in the presence of different concentrations and proportions of two drugs at a time in a checkerboard design. The response parameters were determined, and the sums of the fractional inhibitory concentrations (ΣFICs) of the drug combinations were calculated for different degrees of inhibition (50% effective concentration [EC50], EC90, and EC99). Within therapeutically relevant molar ratios (19 to 200), the combination of quinine and artemisinin showed mean ΣFICs of 1.71 at the EC50, 0.36 at the EC90, and 0.13 at the EC99, indicating increasing synergism. Within the range of molar ratios of 4.3 to 50, the combination of mefloquine and artemisinin yielded mean ΣFCIs of 0.93, 0.44, and 0.31 at the EC50, EC90, and EC99, respectively, indicating synergism. The atovaquone combination showed additive activity to synergism at atovaquone/artemisinin proportions considered relevant to the in vivo situation, i.e., between 4.3 and 200, with the mean ΣFICs decreasing from 1.34 at the EC50 to 0.85 and 0.23 at the EC90 and EC99, respectively. Interstrain differences in the degree of drug interaction were seen with the three strains for all combinations. Synergism was most consistent with quinine.
The evolution and spread of multidrug-resistant Plasmodium falciparum malaria have prompted the need to use combinations of antimalarial drugs, preferably drugs with different modes of action, in order to increase drug efficacy and to potentially reduce the possibility of the development of resistance to either compound. Artemisinin and its derivatives are highly effective compounds against multidrug-resistant P. falciparum, with consistently rapid clearance of parasites and fever in patients with malaria (14). However, in monotherapy with artemisinin and its derivatives, a relatively long treatment course of 5 to 7 days is required; such a treatment course entails the risk of poor compliance and high recrudescence rates (8). Artemisinin therefore needs to be combined with a partner drug with a longer elimination half-life (t1/2) in order to reduce the risk of recrudescence.
In areas with multidrug resistance, i.e., mainly parts of Southeast Asia, the efficacy of mefloquine (as monotherapy) is compromised. Nevertheless, mefloquine and artesunate were found to be synergistic both in vivo and in vitro, and this combination is now widely recommended for clinical use in Southeast Asia (13, 20, 29). However, there is a need for drugs that can be alternative partners to artemisinin, especially in areas where malaria transmission is intensive and where the persistence of subinhibitory concentrations of mefloquine (elimination t1/2, 3 weeks) for long periods entails a high risk of selection of resistant parasite populations (25).
Quinine, a quinoline methanol like mefloquine, represents one potential partner drug to artemisinin. There is relatively little resistance to the drug worldwide, and it has a t1/2 of 9 to 18 h. There is yet little in vitro information and practically no in vivo data on the interactive profile of artemisinin and quinine.
Atovaquone, a hydroxynaphthoquinone with a t1/2 of 52 to 80 h, is a newly introduced antimalarial drug with a novel mode of action. It is well tolerated and active against both chloroquine-sensitive and -resistant strains of P. falciparum, but it should not be used alone for treatment or prophylaxis of malaria. It is formulated as a fixed combination with proguanil since the two drugs have shown a high degree of synergism both in vivo and in vitro (4, 16, 27). One in vitro study has been performed with the combination of artemisinin and atovaquone, but the results were inconclusive (4).
The present study was conducted to assess potential interactions between artemisinin and atovaquone, quinine, or mefloquine and to determine the range of drug concentration ratios for positive pharmacodynamic interactions.
MATERIALS AND METHODS
Parasites and drugs.
Three P. falciparum parasite strains were used for all the in vitro experiments described here. Strains F-32 and FCR-3 and Lab strain 1 (strain K-1) were originally obtained from Tanzania, The Gambia, and Thailand, respectively, but the drug sensitivities of the strains may not reflect their original drug sensitivity at the time of culture adaptation, as is to be expected with polyclonal material. Two strains (strains F32 and FCR3) were originally chloroquine sensitive, and one strain (strain K-1) was partially chloroquine resistant. However, in repeated in vitro tests conducted at the Department of Infectious Diseases, Karolinska Hospital, Stockholm, Sweden, all three strains showed resistance to chloroquine and relatively low levels of sensitivity to quinine and mefloquine. The test drugs included artemisinin, atovaquone, quinine, and mefloquine. Artemisinin was obtained from M. Ashton, Department of Pharmacy, Uppsala University, Uppsala, Sweden; atovaquone base and mefloquine hydrochloride were obtained from Y. Bergqvist, Dalarna University College, Borlänge, Sweden; and quinine hydrochloride was obtained from Ö. Ericsson, Department of Medical Laboratory Sciences and Technology, Karolinska Institute, Stockholm, Sweden.
Parasite cultures.
The three P. falciparum parasite strains were kept in continuous culture by the method of Trager and Jensen (24) in normal RPMI 1640 medium (Gibco BRL, Life Technologies AB, Täby, Sweden). The medium was supplemented with 25 mM HEPES buffer, 2 mg of sodium bicarbonate per ml, 0.5 μg of gentamicin (50 mg/ml) per ml, and 10% human type AB-positive serum. Uninfected human type O-positive erythrocytes were used after they were washed twice with Tris-Hanks' buffer (SBL Vaccine AB, Stockholm, Sweden).
Drug preparations, sensitivity testing, and combination experiments.
All four drugs were dissolved in 95% (vol/vol) alcohol. The solutions were diluted with distilled water to obtain stock solutions of the respective drugs that were 10−3 M.
A series of 10-fold dilutions in water (10−4 to 10−11 M) were prepared from the stock solutions of all drugs. In vitro tests were first run for single drugs, in duplicate, to assess the sensitivities of the three strains to each drug individually. The 50% effective concentrations (EC50s) were determined. Drug solutions were then prepared for the drug interaction experiments on the basis of these estimated EC50s. The concentrations in these final solutions were adjusted to a range between approximately 10−2 and 102 times the EC50s of the respective drugs, using geometric progressions with a factor of 2. These were usually seven or eight different concentrations for each drug.
A checkerboard design (pattern), with single columns for the different test drugs alone and duplicate columns for drug-free controls, was used for the interaction experiments. Artemisinin was included in each experiment and was combined in various concentration ratios with one of the other three drugs. The concentration ranges of artemisinin were between 0.3125 and 80 nM, those for quinine and mefloquine were between 6.25 and 400 nM, and those for atovaquone were between 2.5 and 80 nM. The ranges of molar ratios were 0.16 to 1,240 for quinine/artemisinin and mefloquine/artemisinin and 0.06 to 256 for atovaquone/artemisinin. The drug solutions were introduced into 96-well flat-bottom microtiter plates and were dried prior to the in vitro culture experiments. All experiments were done in duplicate.
In all experiments, (nonsynchronized) cultures started with an initial parasitemia of 0.2 to 0.5% and a hematocrit of 5%. The plates were incubated at 37°C for 48 h in candle jars. Both parasite growth and the degree of inhibition were then estimated by determination of parasite counts (number of infected erythrocytes per 10,000 erythrocytes) on Giemsa-stained thin blood films by light microscopy.
Data analysis.
Since the response of P. falciparum to the test drugs is lognormal, the statistical analysis is based on the log concentration/response probit method, as described by Litchfield and Wilcoxon (15). Drug concentrations were transformed into logarithms, and the percent inhibition values were transformed into probits. The transformed data were processed in a linear least-square regression (9). The various inhibitory concentrations were calculated by the formula logx = (probit y − a)/b, followed by taking anti-logx Growth inhibition at a given drug concentration was calculated by the formula probit y = a + b·logx by entering a given logx and converting the resulting y value from the probit to percent inhibition by using a probit table (1).
The assessment of drug interaction is based on calculation of the sum of the fractional inhibitory concentrations (ΣFICs) at the given EC (3) by the formula (ECx of agent A in the mixture/ECx of agent A alone) + (ECx of agent B in the mixture/ECx of agent B alone).
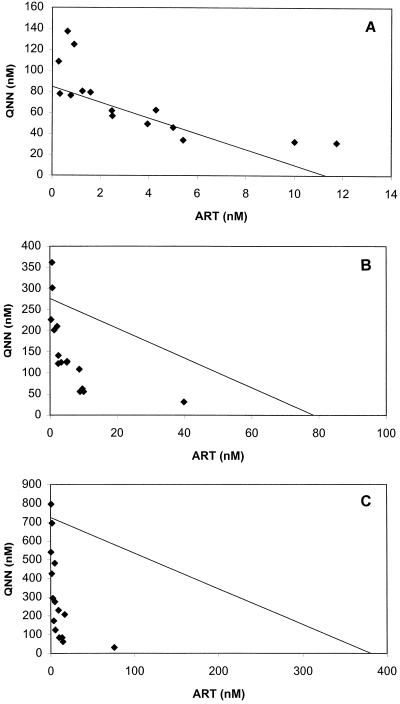
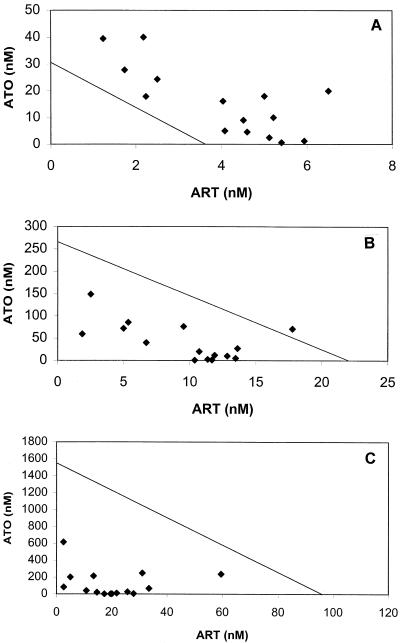
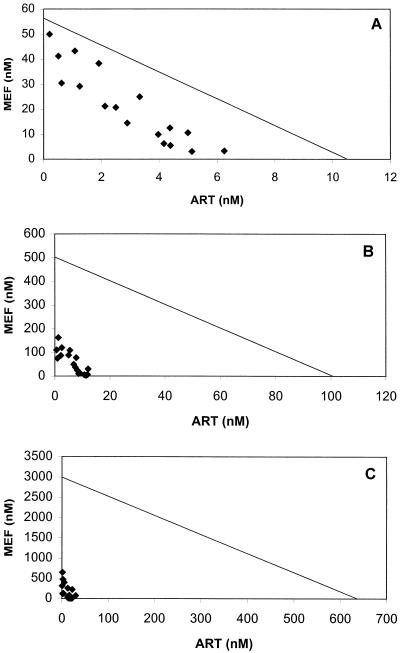
ΣFICs <1 denote synergism, ΣFICs ≥1 and <2 denote additive interaction, ΣFICs ≥2 and <4 denote slight antagonism, and ΣFICs ≥4 denote marked antagonism. ΣFICs <0.5 indicate substantial synergism. In the isobolograms in Fig. 1 to 3, ΣFICs <1 (indicating synergism) appear below the line drawn between the relevant effective concentrations obtained with the single compounds.
FIG. 1.
Isobologram of the interaction between artemisinin (ART) and quinine (QNN) against P. falciparum strain K-1 at the EC50 (A), EC90 (B), and EC99 (C). Datum points below the lines connecting the EC50, EC90, and EC99 for artemisinin and quinine denote synergism; those above the lines denote an ΣFIC ≥1. The EC50, EC90, and EC99 for artemisinin and quinine were derived from log-probit regressions (r = 0.9002 and P < 0.05 for artemisinin; r = 0.9748 and P < 0.05 for quinine)
FIG.3.
Isobologram of the interaction between artemisinin (ART) and atovaquone (ATO) against P. falciparum strain F-32 at the EC50 (A), EC90 (B), and EC99 (C). Datum points below the lines connecting the EC50, EC90, and EC99 for artemisinin and atovaquone denote synergism; those above the lines denote an ΣFIC ≥1. EC50, EC90, and EC99 for artemisinin and atovaquone were derived from log-probit regressions (r = 0.9995 and P < 0.05 for artemisinin; r = 0.9816 and P < 0.05 for atovaquone).
Interpretation in relation to clinically relevant concentration ratios.
In order to project the observed in vitro interactions of the various drug combinations onto the in vivo situation, special consideration was given to the ΣFICs in relation to clinically relevant therapeutic drug concentrations.
The clinically relevant drug ratios were derived from the pharmacokinetic concentration-time profiles, assuming oral coadministration of the medicaments at normal therapeutic doses. After the administration of artemisinin, the maximum concentration of drug in serum (Cmax; ≅1,100 nM) is reached within 1 h; the elimination t1/2 is 1.9 h, and within 6.5 h the drug concentration drops to <100 nM (22). Atovaquone normally reaches Cmax (≅7,600 nM in children; ≅33,000 nM in adults) within 6 h. It has elimination t1/2s of 32 and 60 h in children and adults, respectively. Between 6 and 72 h after the start of treatment, atovaquone concentrations do not drop below 5,000 nM in children and 20,000 nM in adults (2). With the commercial tablet formulations of mefloquine, peak concentrations (4,500 to 11,200 nM) are reached within 8 to 16 h (19); the elimination t1/2 varies between 10 and 21 days. Up to 10 days, the drug concentrations stay well above 2,000 nM. After the oral administration of a loading dose of quinine and after the subsequent administration of normal maintenance doses of quinine, Cmax (≅20,000 nM) is reached within 1 to 3 h, and with dosing every 8 h the concentrations do not drop below 10,000 nM (28).
RESULTS
Determination of the baseline sensitivities to artemisinin of the P. falciparum strains tested indicated geometric mean values for EC50, EC90, and EC99 of 9, 60, and 368 nM, respectively. Strain F-32 was the most sensitive; strain FCR-3 was the least sensitive and showed a flatter concentration/activity regression compared to those for the other two strains. Determination of the baseline sensitivities to quinine showed geometric mean values for EC50, EC90, and EC99 of 139, 873, and 3,908 nM, respectively, with marked interstrain differences in sensitivity being detected. Strain K-1 was the most sensitive, while strain F-32 proved to be the least sensitive. The geometric mean values for EC50, EC90, and EC99 for mefloquine were 56, 181, and 469 nM, respectively, with little variation at the EC50 but marked differences at the EC90 and EC99, at which strain FCR-3 was the least sensitive. The geometric mean values for EC50, EC90, and EC99 for atovaquone were 23, 116, and 438 nM, respectively, with strain F-32 showing a lower level of sensitivity than the other two laboratory strains. The MICs (MIC = EC99) of quinine, mefloquine, and atovaquone were within the concentration range usually observed with normal dosing regimens.
For the combination experiments, the observed values for EC50, EC90, and EC99 were analyzed in relation to the data obtained with the single compounds by using the method of Berenbaum (3), which yields the ΣFICs for each drug combination, strain, and the various ECs. The concentrations of artemisinin and the partner drugs at the EC50, EC90, and EC99 were plotted in isobolograms. The parameters describing the interactions of the combinations of artemisinin and the partner drugs are summarized in Table 1.
TABLE 1.
Interaction between artemisinin and partner drugs against P. falciparum according to parasite strain and effective concentrations
| Partner drug and strain | GM (range) ΣFIC, activitya
|
||
|---|---|---|---|
| EC50 | EC90 | EC99 | |
| Quinine | |||
| F-32 | 3.78 (1.15-29.42), ADD-ANT | 0.35 (0.21-0.60), SYN-SLANT | 0.09 (0.04-1.28), SYN-ADD |
| FCR-3 | 1.45 (0.76-3.79), ADD-SLANT | 0.24 (0.09-0.53), SYN | 0.08 (0.01-0.42), SYN |
| K-1 | 1.11 (0.88-1.68), SYN-ADD | 0.58 (0.32-1.32), SYN-ADD | 0.35 (0.12-1.10), SYN-ADD |
| Mefloquine | |||
| F-32 | 1.08 (0.61-1.96), SYN-ADD | 0.59 (0.07-1.89), SYN-ADD | 1.08 (0.51-2.13), SYN-SLANT |
| FCR-3 | 0.65 (0.51-0.91), SYN | 0.17 (0.11-0.34), SYN | 0.06 (0.03-0.22), SYN |
| K-1 | 1.81 (0.77-2.70), SYN-SLANT | 0.91 (0.58-1.55), SYN-ADD | 0.65 (0.16-2.59), SYN-SLANT |
| Atovaquone | |||
| F-32 | 1.60 (1.20-2.45), ADD-SLANT | 0.57 (0.31-1.07), SYN-ADD | 0.25 (0.08-0.78), SYN |
| FCR-3 | 1.26 (0.95-1.81), SYN-ADD | 1.29 (0.95-1.64), SYN-ADD | 1.28 (0.91-2.56), SYN-SLANT |
| K-1 | 1.13 (0.57-2.80), SYN-SLANT | 0.84 (0.46-12.19), SYN-ANT | 0.41 (0.25-2.05), SYN-SLANT |
GM, geometric mean; SYN, synergistic; ADD, additive; SLANT, slightly antagonistic; ANT, antagonistic.
The activity of the combination of artemisinin and quinine was heterogeneous, but the two drugs predominantly showed additive activity at the EC50. Clear synergism was seen at the EC90 and EC99 (Table 1; see also Table 3). This covered a range of quinine/artemisinin concentration ratios between 0.16 and 1,240. A significant increase in the positive interaction was observed against all strains (Table 2). The interactive profile against strain K-1 is presented in Fig. 1A to C. Synergism was most evident at the EC99.
TABLE 3.
Interaction between artemisinin and partner drugs against P. falciparum at clinically relevant molar ratios (partner drug/artemisinin)
| Partner drug and molar ratio range | GM σFIC (95% CI), activity, ata:
|
||
|---|---|---|---|
| EC50 | EC90 | EC99 | |
| Quinine, 19-200 | 1.71 (1.38-2.13), additive to slightly antagonistic | 0.36 (0.30-0.42), synergistic | 0.13 (0.09-0.18), synergistic |
| Mefloquine, 4.3-50 | 0.93 (0.78-1.11), synergistic-additive | 0.44 (0.33-0.58), synergistic | 0.31 (0.20-0.47), synergistic |
| Atovaquone | |||
| 5-50b | 1.34 (1.22-1.47), additive | 0.85 (0.70-1.03), synergistic-additive | 0.23 (0.18-0.31), synergistic |
| 19-200c | |||
Geometric mean (GM) σFICs and their 95% confidence intervals (CIs; means for three P. falciparum strains).
For children at the second and subsequent doses.
For adults at the second and subsequent doses.
TABLE 2.
Interaction between artemisinin and partner drugs against P. falciparuma
| Partner drug and strain | EC50 vs EC90
|
EC90 vs EC90
|
||||
|---|---|---|---|---|---|---|
| t | P | n | t | P | n | |
| Quinine | ||||||
| F-32 | 29.7278 | <10−10 | 27 | 2.4256 | 0.0220 | 30 |
| FCR-3 | 15.4056 | <10−10 | 36 | 1.2483 | 0.2238 | 36 |
| K-1 | 10.1629 | <10−10 | 30 | 2.4560 | 0.0205 | 30 |
| Mefloquine | ||||||
| F-32 | 3.5327 | 0.0019 | 24 | 6.9628 | <10−7 | 24 |
| FCR-3 | 11.3467 | <10−10 | 34 | 1.3917 | 0.1742 | 34 |
| K-1 | 16.7684 | <10−10 | 34 | 2.5147 | 0.0172 | 34 |
| Atovaquone | ||||||
| F-32 | 27.9378 | <10−10 | 30 | 4.6877 | <10−4 | 30 |
| FCR-3 | 1.1673 | 0.2624 | 27 | 0.1433 | >0.80 | 28 |
| K-1b | 2.0754 | 0.0509 | 21 | 2.7711 | 0.0123 | 21 |
Comparison of the geometric mean ΣFICs by parasite strain and effective concentrations (Student's t test). With the exception of mefloquine for strain F-32, between EC90 and EC99, all differences reflect an increased positive interaction. n, number of isolates.
Comparison of the geometric mean ΣFICs at EC50 and EC99 yielded t = 6.8158 (P < 10−6 at n = 21).
At mefloquine/artemisinin concentration ratios of less than 40, the drugs showed synergistic to additive activities at the EC50. At concentration ratios within the therapeutic range, synergism was most evident against strains FCR-3 and K-1 at the EC90 and EC99 but was less marked against strain F-32, against which a positive interaction was strongest at the EC90. Although the observed ranges of ΣFIC were beyond 1.0 (Table 1), these largely relate to drug ratios outside therapeutic relevance. Significant increases in the positive interaction between the EC50 and the EC90 were evident against all three strains (Table 2). The interactive profile against strain FCR-3 is presented in Fig. 2A to C.
FIG. 2.
Isobologram of the interaction between artemisinin (ART) and mefloquine (MEF) against P. falciparum strain FCR-3 at the EC50 (A), EC90 (B), and EC99 (C). Datum points below the lines connecting the EC50, EC90, and EC99 for artemisinin and mefloquine denote synergism; those above the lines denote an ΣFIC ≥1. The EC50, EC90, and EC99 for artemisinin and mefloquine were derived from log-probit regressions (r = 0.9800 and P < 0.05 for artemisinin; r = 0.9834 and P < 0.05 for mefloquine).
The interaction between artemisinin and atovaquone is predominantly additive at the EC50, but it increases at the EC90 and the EC99 against strains F-32 and K-1, while it remains largely additive against strain FCR-3 (Tables 1 and 2). However, within the therapeutically relevant range of atovaquone/artemisinin concentration ratios, synergism prevails at the EC90 and is quite substantial at the EC99 (Table 3). The interactive profile for strain F-32 is presented in Fig. 3A to C.
The concentration ratios of quinine/artemisinin during a normal combined treatment course are expected to oscillate between 9 and >200, a range that coincides with synergism at EC90 and EC99 (Table 3).
After the first coadministration, the concentration ratios of mefloquine/artemisinin are expected to vary between 0.1 and 24; after the subsequent administration of artemisinin, they oscillate between 4.3 and 50. This is within a synergistic range at the EC90 and the EC99 (Table 3).
During the first coadministration of atovaquone and artemisinin the concentration ratios are likely to rise from 0.3 to 50 in children and from 1.5 to 200 in adults. With subsequent drug doses, the range of atovaquone/artemisinin ratios will vary between 4.8 and 50 in children and between 19 and 200 in adults. At the EC90 this is mostly in the synergistic range; at the EC99 this fully in the synergistic range (Table 3).
DISCUSSION
The most widely used artemisinin derivatives are artemether and sodium artesunate. Their main active principle is dihydroartemisinin, the metabolite common to artemisinin and its derivatives. In these studies artemisinin was used in preference over dihydroartemisinin since it is more stable than the metabolite. Moreover, the activities of artemisinin and dihydroartemisinin show high degrees of correlation (26). Another reason for the use of artemisinin is its pharmacokinetic profile after oral administration, which is quite similar to that of artemether but which is longer than those of sodium artesunate and dihydroartemisinin.
The EC50s and EC90s of artemisinin, mefloquine, and quinine for the three strains were about the same magnitude or lower than those observed for fresh P. falciparum isolates (26) or recently culture-adapted strains from Thailand (30, 31). The interaction between artemisinin and atovaquone, quinine, or mefloquine at the EC50 was usually in the additive range, while it showed synergism at the EC90, and the synergism was even more marked at the EC99. However, qualitatively and quantitatively, the interactions between artemisinin and the partner drugs showed considerable interstrain differences, highlighting the importance of obtaining observations for a larger number of fresh P. falciparum isolates.
The phenomenon of a positive concentration-dependent pharmacodynamic interaction that improves with an increase in the concentration, seen particularly with quinine and atovaquone, may be due to the relative impact of the drug on parasite survival at the given ECs. It is likely that the life functions of the surviving parasites are also compromised and that this effect becomes more marked with increasing drug concentrations. A synergistic interaction becomes more plausible under such conditions of increasing fragility.
It may be argued that eventual synergism between the artemisinin compounds and partner drugs with longer t1/2s will be irrelevant in a practical sense in view of the rather short t1/2s of the former. This would apply to artesunate and dihydroartemisinin (t1/2, <30 min) more than to artemisinin (t1/2, ≈2 h; duration of significant interaction, ≥6 h). Artemisinin and its derivatives reduce a large proportion of the parasite population (usually about 99%) within one exposure, which covers a relatively short time span. Although the time available for interaction with other drugs will be limited, it is likely that such an interaction will occur, especially when the partner drug has been present for some time and has had the opportunity to massively reduce the vitality of the parasite population.
The combination of atovaquone and artemisinin showed additive activity to synergism against the three P. falciparum strains. The synergism was most evident at atovaquone/artemisinin concentration ratios >16.5, which fell into the clinically relevant range (10, 21, 23). In a previous study, both synergistic and antagonistic interactions were found between atovaquone and artemisinin (4). In the absence of an explanation for these contradictory results, it can be concluded that there is some evidence of synergism between the two drugs; but the clinical importance remains unclear until the results of in vivo studies will become available.
The combination of quinine and artemisinin showed clear synergism over a wide range of concentration ratios. The synergy was found to be most prominent at quinine/artemisinin concentration ratios <35, which are usually present for approximately 2 h, which is near the time of the Cmax of artemisinin. Previous in vitro studies on the combinations artesunate-quinine and arteether-quinine have also shown that they are synergistic (11, 13). Quinine shares structural similarities with mefloquine, but it is not known whether the mechanism of action of quinine is more closely related to that of chloroquine or mefloquine. Quinine inhibits heme polymerization within the food vacuole (22). Artemisinin is also reported to react with hemin within the food vacuole. Therefore, these drugs might act synergistically at different sites of the same target molecule. Since quinine has a relatively short t1/2 (8.7 h in healthy subjects and up to 18.2 h in severely ill patients), the therapeutic regimen consists of doses every 8 h for 7 days. In areas with multidrug resistance, monotherapy with quinine is rarely curative, but in analogy to the combination of mefloquine and artemisinin derivatives, it may be expected that medication with quinine and artemisinin or one of its derivatives in combination would effect the elimination of the infection.
The combination of artemisinin and mefloquine showed synergism at mefloquine/artemisinin ratios <40. These represent most of the clinically relevant ranges of the concentration ratios of both drugs. Both in vitro and in vivo studies with combinations of artemisinins with mefloquine have shown synergism (5, 6, 11, 13), and the mefloquine-artesunate combination is already in clinical use in areas of Thailand with multidrug-resistant parasites (17). The synergistic behavior may be explained by several factors. First, it is believed that heme itself or heme polymerase within the food vacuole is the target of the quinoline derivatives (7, 22). Artemisinin has also been reported to react with hemin within the food vacuole (18). Second, mefloquine binds with a high affinity to membrane phospholipids, and artemisinin interacts with the mitochondrial membrane and other membranes. Thus, it would be plausible that the interaction of artemisinin with the parasite membrane enhances the effect of mefloquine (7, 12). The period of treatment with this combination is 2 to 3 days. However, because of the relatively poor tolerability and the long elimination t1/2 of mefloquine, there appears to be a place for partner drugs other than artemisinin. The residual subinhibitory mefloquine concentrations entail a high risk of selection and occurrence of resistance, especially in areas where malaria transmission is intensive.
Since quinine and mefloquine both belong to the quinoline methanol class and both show synergistic activity with artemisinin, it may be concluded that artemisinins are synergistic with the quinoline methanol group of compounds and, possibly, with most, if not all, other members of the class 2 aryl amino alcohol antimalarial compounds. The degree of synergism between artemisinin and mefloquine and between artemisinin and quinine was found to be similar to that reported from earlier in vitro studies of the artemether-benflumetol combination (1).
With all the limitations inherent in projecting in vitro findings onto the clinical in vivo condition, the results of our studies seem to confirm the fact that mefloquine is a good option for use in combination with artemisinin, especially in areas with low rates of malaria transmission, but quinine may be considered for multidrug-resistant areas where malaria transmission is intensive.
The data suggest that the practical use of the synergism between artemisinin and partner drugs could be optimized by the appropriate timing of administration of the artemisinin doses, in accordance with the pharmacokinetic characteristics of the partner drug.
Moreover, it would be interesting to extend the in vitro interaction studies to dihydroartemisinin, whenever feasible, with exposure conditions that simulate those of the in vivo situation. However, before embarking on such a study it would be wise to investigate the concentration profile of dihydroartemisinin in the incubated cell-medium mixture since there is the distinct possibility of a time-dependent reduction to an inactive analogue.
Acknowledgments
This work was financially supported by grants from the Swedish International Development Agency (Sida/SAREC).
REFERENCES
- 1.Alin, M. H., A. Bjorkman, and W. H. Wernsdorfer. 1999. Synergism of benflumetol and artemether in Plasmodium falciparum. Am. J. Trop. Med. Hyg. 61:439-445. [DOI] [PubMed] [Google Scholar]
- 2.Beerahee, W. 1999. Clinical pharmacology of atovaquone and proguanil hydrochloride. J. Travel Med. 6:S13-S17. [PubMed] [Google Scholar]
- 3.Berenbaum, M. C. 1978. A method for testing synergy with any number of agents. J. Infect. Dis. 137:122-130. [DOI] [PubMed] [Google Scholar]
- 4.Canfield, C. J., M. Pudney, and W. E. Gutteridge. 1995. Interactions of atovaquone with other antimalarial drugs against Plasmodium falciparum in vitro. Exp. Parasitol. 80:373-381. [DOI] [PubMed] [Google Scholar]
- 5.Chawira, A. N., and D. C. Warhurst. 1987. The effect of artemisinin combined with standard antimalarials against chloroquine-sensitive and chloroquine-resistant strains of Plasmodium falciparum in vitro. J. Trop. Med. Hyg. 90:1-8. [PubMed] [Google Scholar]
- 6.Chawira, A. N., D. C. Warhurst, B. L. Robinson, and W. Peters. 1987. The effect of combinations of qinghaosu (artemisinin) with standard antimalarial drugs in the suppressive treatment of malaria in mice. Trans. R. Soc. Trop. Med. Hyg. 81:554-558. [DOI] [PubMed] [Google Scholar]
- 7.Chevli, R., and C. D. Fitch. 1982. The antimalarial drug mefloquine binds to membrane phospholipids. Antimicrob. Agents Chemother. 21:581-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.China Cooperative Research Group on Qinghaosu and Its Derivatives as Antimalarials. 1982. Metabolism and pharmacokinetics of qinghaosu and its derivatives. J. Traditional Chinese Med. 2:25-30. [PubMed] [Google Scholar]
- 9.Daniel, W. W. 1991. A foundation for analysis in the health sciences, 5th ed. John Wiley & Sons, Inc., New York, N.Y.
- 10.Duc, D. D., P. J. de Vries, X. K. Nguyen, B. Le Nguyen, P. A. Kager, and C. J. van Boxtel. 1994. The pharmacokinetics of a single dose of artemisinin in healthy Vietnamese subjects. Am. J. Trop. Med. Hyg. 51:785-790. [DOI] [PubMed] [Google Scholar]
- 11.Ekong, R., and D. C. Warhurst. 1990. Synergism between arteether and mefloquine or quinine in a multidrug-resistant strain of Plasmodium falciparum in vitro. Trans. R. Soc. Trop. Med. Hyg. 84:757-758. [DOI] [PubMed] [Google Scholar]
- 12.Ellis, D. S., Z. L. Li, H. M. Gu, W. Peters, B. L. Robinson, G. Tovey, and D. C. Warhurst. 1985. The chemotherapy of rodent malaria. XXXIX. Ultrastructural changes following treatment with artemisinine of Plasmodium berghei infection in mice, with observations of the localization of [3H] dihydroartemisinine in P. falciparum in vitro. Ann. Trop. Med. Parasitol. 79:367-374. [PubMed] [Google Scholar]
- 13.Fivelman, Q. L., J. C. Walden, P. J. Smith, P. I. Folb, and K. I. Barnes. 1999. The effect of artesunate combined with standard antimalarials against chloroquine-sensitive and chloroquine-resistant strains of Plasmodium falciparum in vitro. Trans. R. Soc. Trop. Med. Hyg. 93:429-432. [DOI] [PubMed] [Google Scholar]
- 14.Hien, T. T., and N. J. White. 1993. Qinghaosu. Lancet 341:603-608. [DOI] [PubMed] [Google Scholar]
- 15.Litchfield, J. T., and F. Wilcoxon. 1949. A simplified method of evaluating dose-effect experiments. J. Pharmacol. Exp. Ther. 96:99-113. [PubMed] [Google Scholar]
- 16.Looareesuwan, S., C. Viravan, H. K. Webster, D. E. Kyle, D. B. Hutchinson, and C. J. Canfield. 1996. Clinical studies of atovaquone, alone or in combination with other antimalarial drugs, for treatment of acute uncomplicated malaria in Thailand. Am. J. Trop. Med. Hyg. 54:62-66. [DOI] [PubMed] [Google Scholar]
- 17.Looareesuwan, S., P. Wilairatana, W. Chokejindachai, P. Viriyavejakul, S. Krudsood, and P. Singhasivanon. 1998. Research on new antimalarial drugs and the use of drugs in combination at the Bangkok Hospital for Tropical Diseases. Southeast Asian J. Trop. Med. Public Health 29:344-354. [PubMed] [Google Scholar]
- 18.Meshnick, S. R., A. Thomas, A. Ranz, C. M. Xu, and H. Z. Pan. 1991. Artemisinin (qinghaosu): the role of intracellular hemin in its mechanism of antimalarial action. Mol. Biochem. Parasitol. 49:181-189. [DOI] [PubMed] [Google Scholar]
- 19.Na-Bangchang, K., J. Karbwang, P. A. Palacios, R. Ubalee, S. Saengtertsilapachai, and W. H. Wernsdorfer. 2000. Pharmacokinetics and bioequivalence evaluation of three commercial tablet formulations of mefloquine when given in combination with dihydroartemisinin in patients with acute uncomplicated falciparum malaria. Eur J. Clin. Pharmacol. 55:743-748. [DOI] [PubMed] [Google Scholar]
- 20.Nosten, F., and R. N. Price. 1995. New antimalarials. A risk-benefit analysis. Drug Safety 12:264-273. [DOI] [PubMed] [Google Scholar]
- 21.Rolan, P. E., A. J. Mercer, E. Tate, I. Benjamin, and J. Posner. 1997. Disposition of atovaquone in humans. Antimicrob. Agents Chemother. 41:1319-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slater, A. F., and A. Cerami. 1992. Inhibition by chloroquine of a novel haem polymerase enzyme activity in malaria trophozoites. Nature 355:167-169. [DOI] [PubMed] [Google Scholar]
- 23.Titulaer, H. A., J. Zuidema, P. A. Kager, J. C. Wetsteyn, C. B. Lugt, and F. W. Merkus. 1990. The pharmacokinetics of artemisinin after oral, intramuscular and rectal administration to volunteers. J. Pharm. Pharmacol. 42:810-813. [DOI] [PubMed] [Google Scholar]
- 24.Trager, W., and J. B. Jensen. 1976. Human malaria parasites in continuous culture. Science 193:673-675. [DOI] [PubMed] [Google Scholar]
- 25.Wernsdorfer, W. H., T. Chongsuphajaisiddhi, and N. P. Salazar. 1994. A symposium on containment of mefloquine-resistant falciparum malaria in Southeast Asia with special reference to border malaria. Southeast Asian J. Trop. Med. Public Health 25:11-18. [PubMed] [Google Scholar]
- 26.Wernsdorfer, W. H., G. Wernsdorfer, S. Prajakwong, B. Woitsch, C. Zatloukal, and H. Kollaritsch. 2000. Activity correlation between artemisinin and dihydro-artemisinin in fresh isolates of Plasmodium falciparum from Thailand. Mitteil. Öesterreich. Ges. Tropenmed. Parasitol. 22:87-94. [Google Scholar]
- 27.White, N. J. 1999a. Antimalarial drug resistance and combination chemotherapy. Philos. Trans. R. Soc. London Ser. B Biol. Sci. 354:739-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White, N. J. 1987. The pharmacokinetics of quinine and quinidine in malaria. Acta Leiden 55:65-76. [PubMed] [Google Scholar]
- 29.Wilairatana, P., S. Krudsood, W. Chokejindachai, V. Bussaratid, U. Silachamroon, P. Viriyavejakul, C. Hendriksen, M. W. Scheiwe, and S. Looareesuwan. 1998. A clinical trial of combination of artesunate and mefloquine in the treatment of acute uncomplicated falciparum malaria: a short and practical regimen. Southeast Asian J. Trop. Med. Public Health 29:696-701. [PubMed] [Google Scholar]
- 30.Wongsrichanalai, C., T. Wimonwattrawatee, P. Sookto, A. Laoboonchai, D. G. Heppner, D. E. Kyle, and W. H. Wernsdorfer. 1999. In vitro sensitivity of Plasmodium falciparum to artesunate in Thailand. Bull. W. H. O. 77:392-398. [PMC free article] [PubMed] [Google Scholar]
- 31.Wongsrichanalai, C., K. Thimasarn, and, J. Sirichaisinthop. 2000. Antimalarial drug combination policy: a caveat. Lancet 355:2245-2247. [DOI] [PubMed] [Google Scholar]